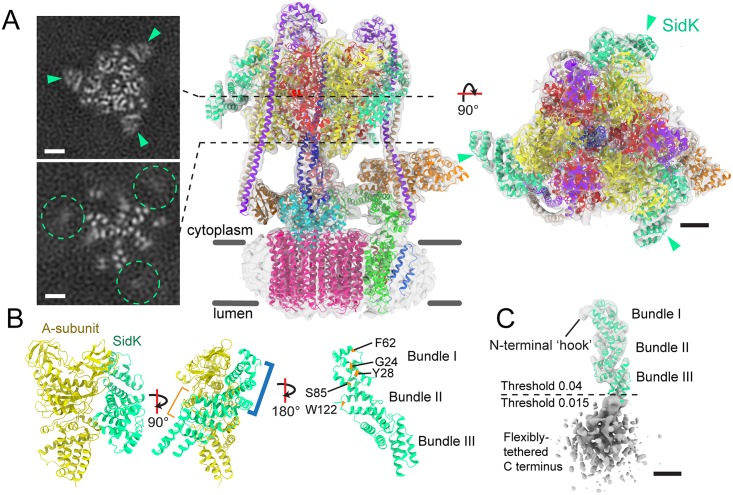
Fig 2. Cryo-EM structure of the V-ATPase:SidK3 complex.
A, Three SidK proteins (teal) are bound to the V-ATPase in the soluble catalytic V1 region. The SidK density is well-defined in the N-terminal region where it binds the V-ATPase (teal arrowheads) and is poorly-resolved in the C-terminal region closer to the membrane-embedded part of the complex (teal dashed circles). B, SidK binds to the N-terminal region of subunit A of the V-ATPase. The major interaction surface is on SidK α-helical bundle I (blue bracket) and appears to involve residues G24, Y28, F62, S85, and W122. C, The cryo-EM map density (gray surface) reveals a feature resembling a 'hook' at the N terminus of SidK that is missing from the crystal structure. The C-terminal region of SidK is flexibly-tethered to its N-terminal region and is poorly resolved in the cryo-EM density. For clarity, the surface representation of SidK was rendered at a higher density threshold for the N-terminal domain (Threshold 0.04) than for the C-terminal domain (Threshold 0.015). Scale bars: 25 Å.