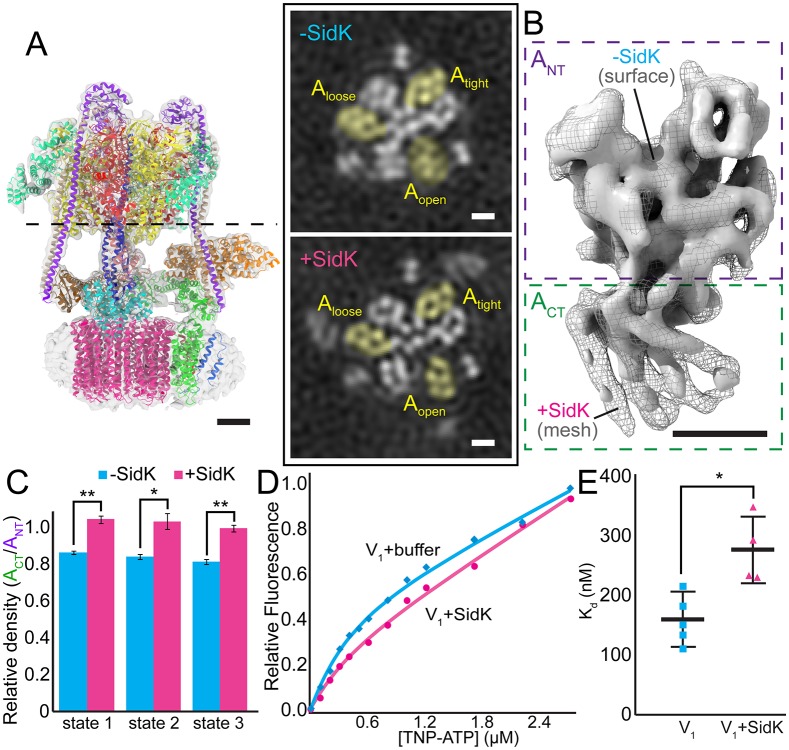
Fig 4. SidK reduces the flexibility of the A-subunit and its binding affinity for TNP-ATP.
A, Slices through the cryo-EM maps of V-ATPase and V-ATPase:SidK3 in state 3, low-pass filtered to 10 Å. In V-ATPase without SidK (-SidK), the C-terminal region of the A-subunit (ACT) in the ‘open’ conformation (Aopen) has lower density compared to the A-subunit in the ‘tight’ or ‘loose’ conformations (Atight, Aloose). With SidK bound (+SidK), density for the ‘open’ A-subunit is comparable to other subunits in the V-ATPase:SidK3 map. B, Comparison between the A-subunits in the ‘open’ conformation from V-ATPase (gray surface) and V-ATPase:SidK3 (gray mesh) cryo-EM maps. ACT is better defined with SidK bound. Scale bars: 25 Å. C, The average density of ACT relative to ANT is higher with SidK bound to V-ATPase in all rotational states. D, Representative relative fluorescence values and fitted curves from titration of TNP-ATP into a solution containing V1 alone (blue) or V1 with SidK (pink). E, Dissociation constant Kd values calculated from data in (D) show increased Kd values with SidK treatment, indicating that SidK binding decreases the affinity of the V1 subcomplex for TNP-ATP. *, p<0.01; **, p<0.001.