This study presents results of the BALLET study, which focused on the use of everolimus/exemestane combination in postmenopausal women with ER+ locally advanced or metastatic breast cancer after recurrence or progression after nonsteroidal aromatase inhibitor treatment. Real‐life data of the cohort are reported.
Keywords: Advanced breast cancer, Safety, Everolimus, Hormone‐receptor positive, Real life
Abstract
Background.
The BALLET study was an open‐label, multicenter, expanded access study designed to allow treatment with everolimus plus exemestane in postmenopausal women with hormone receptor‐positive metastatic breast cancer progressed following prior endocrine therapy. A post hoc analysis to evaluate if previous chemotherapy in the metastatic setting affects the safety profile of the combination regimen of everolimus and exemestane was conducted on the Italian subset, as it represented the major part of the patients enrolled (54%).
Patients and Methods.
One thousand one hundred and fifty‐one Italian patients were included in the present post hoc analysis, which focused on two sets of patients: patients who never received chemotherapy in the metastatic setting (36.1%) and patients who received at least one chemotherapy treatment in the metastatic setting (63.9%).
Results.
One thousand one hundred and sixteen patients (97.0%) prematurely discontinued the study drug, and the main reasons reported were disease progression (39.1%), local reimbursement of everolimus (31.1%), and adverse events (AEs) (16.1%). The median duration of study treatment exposure was 139.5 days for exemestane and 135.0 days for everolimus. At least one AE was experienced by 92.5% of patients. The incidence of everolimus‐related AEs was higher (83.9%) when compared with those that occurred with exemestane (29.1%), and the most commonly reported everolimus‐related AE was stomatitis (51.3%). However, no significant difference in terms of safety related to the combination occurred between patients without and with chemotherapy in the metastatic setting.
Conclusion.
Real‐life data of the Italian patients BALLET‐related cohort were an adequate setting to state that previous chemotherapy did not affect the safety profile of the combination regimen of everolimus and exemestane.
Implications for Practice.
With the advent of new targeted agents for advanced or metastatic breast cancer, multiple lines of therapy may be possible, and components of the combined regimens can overlap from one line to another. Thus, it is important to assess even the potential of cumulative and additive toxic effects among the drugs. Previous chemotherapy did not affect the safety profile of the combination regimen of everolimus and exemestane. The continuous monitoring of the safety signals of this drug combination from general clinical practice is important, in particular for stomatitis.
摘要
背景. BALLET研究是一项开放性、多中心、扩大供药研究, 患有激素受体阳性转移性乳腺癌且既往内分泌治疗后疾病进展的绝经后女性可在研究中接受依维莫司和依西美坦联合治疗。入组患者大部分为意大利人(54%), 因此在意大利患者子集中进行了一项事后分析, 以评价既往在疾病转移后接受化疗是否会影响依维莫司和依西美坦联合治疗方案的安全性特征。
患者和方法. 本项事后分析纳入了1 151例意大利患者, 重点分析两组患者:从未接受过化疗的转移性乳腺癌患者(36.1%)和至少接受过一次化疗的转移性乳腺癌患者(63.9%)。
结果. 1 116例患者(97.0%)提前停用研究药物, 报告的主要原因为疾病进展(39.1%)、当地报销依维莫司(31.1%)和不良事件(AE;16.1%)。依西美坦和依维莫司的研究治疗中位持续时间分别为139.5天和135.0天。92.5%的患者至少发生1例AE。依维莫司(83.9%)相关AE的发生率高于依西美坦(29.1%), 最常报告的依维莫司相关AE为口腔炎(51.3%)。然而, 接受和未接受化疗的转移性乳腺癌患者在联合治疗的相关安全性方面没有显著差异。
结论. BALLET研究中意大利患者队列的实际数据充分说明, 既往化疗不影响依维莫司和依西美坦联合治疗方案的安全性特征。The Oncologist 2017;22:648–654
对临床实践的提示:晚期或转移性乳腺癌的新靶向药物问世后, 患者或许可以接受多线治疗, 而各线联合治疗方案中的药物可能相互重叠。因此, 评估药物毒性作用的潜在累积和叠加风险至关重要。既往化疗不影响依维莫司和依西美坦联合治疗方案的安全性特征。在一般临床实践中必须持续监测这一联合用药的安全性信号, 特别是口腔炎。
Introduction
Endocrine therapy is an important class of target‐directed therapy blocking the growth‐promoting effects of estrogen via estrogen receptors (ER) [1]. Although endocrine therapy continues to be the cornerstone of effective treatment of ER‐positive (ER+) breast cancer (BC), the emergence of the resistance to endocrine therapy is frequent [2]. Intensive research has identified a number of potentially targetable pathways that interact with ER signaling in BC leading to cancer progression beyond endocrine receptor blockade [3], [4], [5], [6]; one mechanism implicated in endocrine resistance in BC is the activation of the phosphatidylinositol 3‐kinase/protein kinase B/mammalian target of rapamycin (mTOR) signal transduction pathway [7], [8]. Based on this hypothesis, the Breast Cancer Trials of Oral Everolimus‐2 (BOLERO‐2) phase III study had been conceived to evaluate the efficacy and the safety of the combination of everolimus (an mTOR inhibitor) and exemestane (steroidal aromatase inhibitor) in patients with ER+ BC progressed to nonsteroidal aromatase inhibitors (NSAIs) [9]. The BOLERO‐2 results showed that the addition of everolimus to endocrine therapy leads to an improved clinical outcome with significant PFS benefit. Conclusions also stated that careful monitoring of patients and increased physician awareness of the safety profile of everolimus‐based therapy were warranted.
The BALLET study was a European open‐label, multicenter, expanded access study designed to allow treatment with everolimus plus exemestane in postmenopausal women with ER+ locally advanced or metastatic BC who have progressed following prior NSAIs. Safety results of this study, recently published, enabled further investigation of this drug combination in a clinical setting mimicking the real world [10]. The aim of the present report is to present and discuss the post hoc analysis performed on Italian patients enrolled in the BALLET study (1,153 out of 2,131 of the whole study population), reporting results in everyday clinical practice evaluating the possible differences on the cumulative toxicity of everolimus plus exemestane in patients who previously received or did not received chemotherapy in the metastatic setting.
Materials and Methods
The BALLET study was a European, multicenter, open‐label, single‐arm, phase III b, expanded access study focused on the use of everolimus/exemestane combination in postmenopausal women with ER+ locally advanced or metastatic BC after recurrence or progression following NSAIs treatment (EudraCT Number: 2012‐000073‐23, CRAD001YIC04). Complete inclusion/exclusion criteria list, study design, and treatment were already reported [10]. Everolimus was provided from May 2012 until the drug was locally reimbursed for this indication or until 31 January 2014. The global study enrolled 2,131 patients, of whom 1,153 (54.1%) were Italian (128 cancer centers). Local reimbursement in Italy has been granted since July 2013.
The primary study objective was to evaluate safety of everolimus plus exemestane in postmenopausal women with ER+ locally advanced or metastatic BC after recurrence or progression following NSAIs treatment. The assessment of safety was based mainly on the frequency of adverse events (AEs) and on the number of laboratory values that were new or worsening. Vital signs, Eastern Cooperative Oncology Group (ECOG) performance status, and physical examination were also assessed during the study period. The secondary objective was to evaluate grade 3 and 4 AEs during treatment with everolimus and exemestane in the routine clinical practice (frequency of AEs recorded as grade ≥3 or as serious A E [SAE]). Stomatitis and pneumonitis were considered events of particular interest as the most frequent infections reported in the BOLERO‐2 trial and the most frequently reported AEs that led to permanent treatment discontinuation in the BALLET study [9], [10]. The BALLET study did not employee stomatitis prevention methodology.
The present analysis performed on the Italian cohort of the BALLET study was focused on the following two subpopulations: patients without initial chemotherapy treatment prior to everolimus/exemestane combination (“without‐chemo group”) and patients with initial chemotherapy treatment prior to everolimus/exemestane combination (“with‐chemo group”). In particular, the subpopulation with no initial chemotherapy included only patients who never received chemotherapy in the metastatic setting, whereas the subpopulation with initial chemotherapy included only patients who received at least one chemotherapy treatment in the metastatic setting, whatever the line of treatment.
The safety population included all patients who received at least one dose of everolimus and exemestane and had at least one post‐baseline safety assessment. The mean cumulative dose (the total dose given during the study treatment exposure), the mean dose intensity (the ratio between cumulative dose and duration of exposure), and compliance to dose regimen planned by protocol (relative dose intensity, i.e., the ratio between the actual dose intensity and the planned dose intensity) were calculated. All the AEs were assessed by the Common Terminology Criteria (CTCAE), version 4.03.
The study was approved by the ethical committee at each site and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. All the patients provided written informed consent.
Patients
One thousand two hundred and seventy‐nine (1,279) female Italian patients were screened, 1,153 (90.1% of the screened set) of these were enrolled, and 1,151 (90.0% of the screened set) were included in the present analysis. Two patients were excluded due to lack of baseline data. Patients who never received chemotherapy in the metastatic setting were 416 (36.1%) and those who received at least one chemotherapy treatment in the metastatic setting were 735 patients (63.9%).
Patients’ baseline characteristics are summarized in Table 1. Briefly, median age at treatment was 64 years (range 33–85 years), 47.9% were aged ≥65 years, and 29.1% were aged ≥70 years; 98.7% were white and 97.5% had an ECOG performance status ≤1. Body mass index was 26.1 kg/m2 on average.
Table 1. Baseline and disease characteristics.
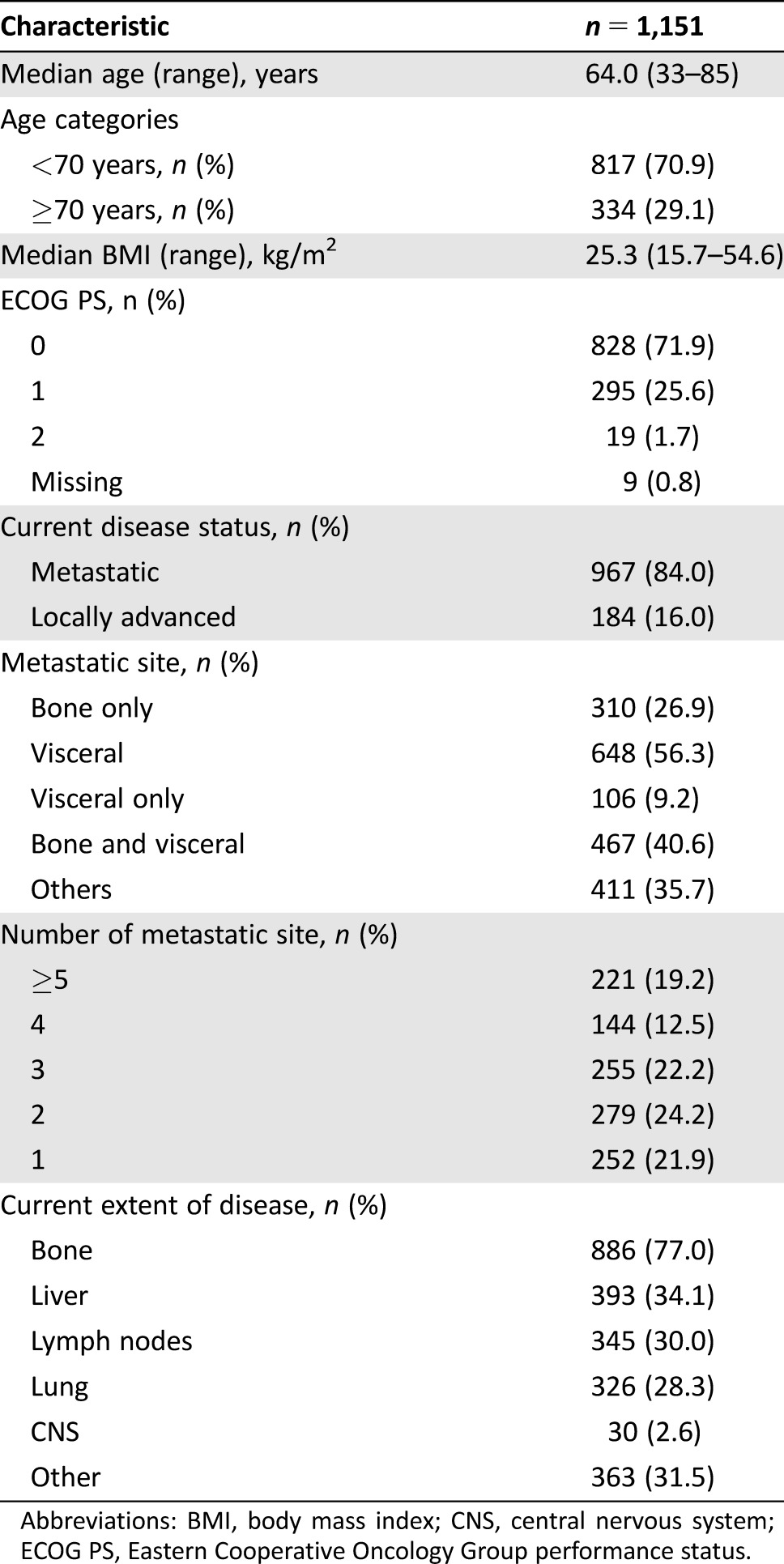
Abbreviations: BMI, body mass index; CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group performance status.
Seventy‐seven percent of study population (n = 886) had an extent of disease involving bone and 34.1% (n = 393) involving liver. The majority of the patients of both groups had a metastatic cancer (85.1% in the without‐chemo group versus 83.4% in the with‐chemo group). For both groups, the most frequent metastatic site of cancer was visceral (43.8% in the without‐chemo group versus 63.4% in the with‐chemo group); 49.3% of the patients in the without‐chemo group and 56.5% of the patients in the with‐chemo group had at least three metastatic sites of cancer involved.
Mean time elapsed from diagnosis to the informed consent signature was approximately 10 years. Prior antineoplastic medications were administered mainly in therapeutic (88.0%) and adjuvant (79.0%) settings. One hundred twenty‐five patients (10.9%) received prior antineoplastic medications in the adjuvant setting only, while 23.5% underwent at maximum until first line of treatment in advance setting, 21.3% until second line, 15.9% until third line, 10.5% until fourth line, and 17.8% until fifth line or over.
Treatment
The mean duration of study treatment exposure was 158.3 ± 106.8 days (median 139.5) for exemestane and 153.9 ± 108.5 days (median 135.0) for everolimus, ranging between 1 and 706 days. Two hundred forty‐five (21.3%) and 712 (61.9%) patients temporary interrupted exemestane and everolimus, respectively, for an average of 14.1 days for exemestane and 24.2 days for everolimus. Three hundred thirty (28.7%) patients took everolimus at a 5‐mg dose for a mean of 99.8 days, and almost all patients (1,149, 99.8%) took everolimus at the prescribed 10‐mg dose for a mean of 113.7 days.
The mean cumulative dose was 3,900.4 ± 2,644.6 mg for exemestane and 1,279.3 ± 919.7 mg for everolimus, while the mean dose intensity was 24.6 ± 1.2 mg/day for exemestane and 8.6 ± 1.8 mg/day for everolimus. Relative dose intensity (patients'compliance) resulted on average 0.98 ± 0.05 for exemestane and 0.86 ± 0.18 for everolimus. Treatment compliance was higher on exemestane than everolimus; the percentage of patients with compliance higher than 90% was 94.4% and 58.6% for exemestane and everolimus, respectively; the percentage of patients with compliance lower than 60% was 0.1% and 15.1% for exemestane and everolimus, respectively. No difference in treatment exposure was noticed between the two subgroups.
One thousand twenty‐nine (89.4%) took at least one concomitant medication/significant nondrug therapy administered during the study and for up to 28 days after study drug discontinuation. The most reported concomitant medication was zoledronic acid (390 patients, 33.9%).
Sixty‐two percent of patients were administered at least one antineoplastic medication since discontinuation of study drug (64.4% in the without‐chemo group versus 60.0% in the with‐chemo group). The most reported antineoplastic medication was exemestane (292 patients, 25.4%).
Safety
Sixty‐nine patients (6.0%) died during the study or within 28 days after last study treatment dose. The main causes of deaths were disease or tumor progression (n = 31), (S)AEs (n = 17), worsening of general conditions (n = 7), sudden death (n = 7), and other causes (n = 7).
In terms of serious/clinically significant AEs, 209 patients (18.2%) experienced at least one SAE (0.2% experienced a SAE leading to hospitalization or prolongation of hospitalization) and 155 (13.5%) experienced at least one AE leading to discontinuation of everolimus or exemestane. The incidence of fatal and significant AE was slightly higher—but not statistically significant—in the group of patients with chemotherapy in the metastatic setting compared with the group of patients without chemotherapy in the metastatic setting, in particular 8.0% versus 2.4% for deaths, 20.4% versus 14.2% for SAEs, and 14.6% versus 11.5% for AEs leading to treatment discontinuation, respectively.
Incidence of grade 3 and 4 AEs leading to permanent discontinuation of study treatment was 11.0% (9.6% in the without‐chemo group versus 11.8% in the with‐chemo group in the metastatic setting). The most frequent grade 3 and 4 events leading to permanent discontinuation of treatment were stomatitis (2.8%), anemia (1.7%), hyperglycemia (1.1%), and asthenia (1.0%).
The most reported grade 3 or 4 toxicities were observed for hematologic parameters in absolute lymphocytes, which decreased for 50 patients (4.3%), and in WBC, which decreased for 10 patients (0.9%), and for biochemistry parameters, for 123 patients in gamma glutamyltransferase (10.7%) and for 64 patients in glucose (5.6%). Overall, 93% of patients experienced at least one AE, in particular 91.1% of patients without chemotherapy in the metastatic setting and 93.3% of patients with chemotherapy in the metastatic setting without significant difference in the two subgroups (Table 2).
Table 2. Incidence of adverse events in either group (at least 10%).
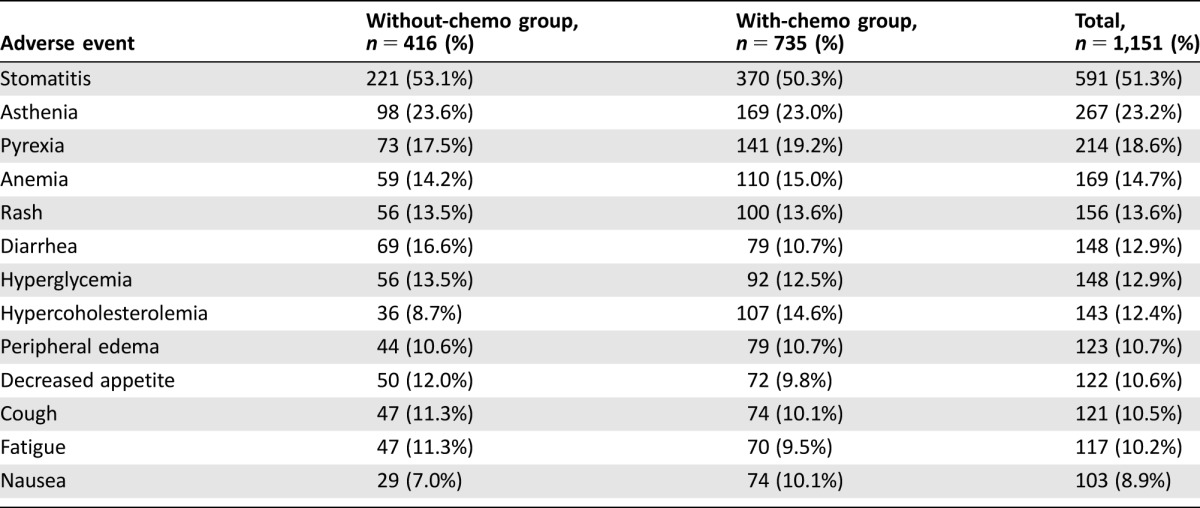
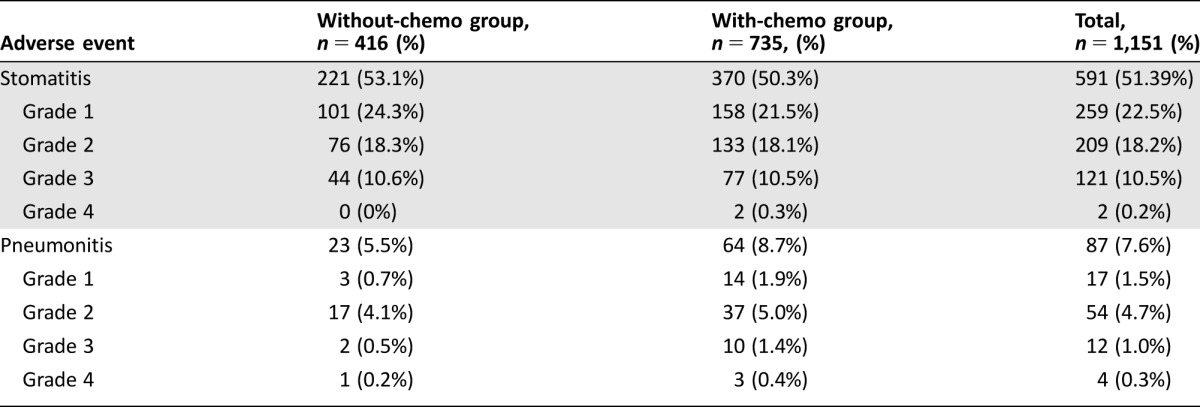
The most reported AE was stomatitis (51.3%). The observed incidence of stomatitis by severity was 22.5% for CTCAE grade 1, 18.2% for grade 2, 10.5% for grade 3, and 0.2% for grade 4. Pneumonitis had a lower incidence: 7.6% of patients experienced at least one event (1.5%, 4.7%, 1.0%, and 0.3% split by CTCAE grades 1, 2, 3, and 4, respectively; Table 3). The probability of experiencing stomatitis at 6 months from the start of treatment was approximately 50%, slightly less than the 10% probability for pneumonitis.
Table 3. Incidence and severity of stomatitis and pneumonitis.
The incidence of everolimus‐related AEs was higher compared with the occurrence of exemestane‐related events; 83.9% of patients (both in without‐ and with‐chemo groups) experienced at least one AE related to everolimus, whereas 29.1% (26.7% versus 30.5% in without‐ and with‐chemo groups) experienced at least one AE related to exemestane. The most reported AE related to everolimus was stomatitis, which was experienced at least once by approximately half (49.7%) of the patients overall.
Weight loss (defined as decrease from baseline of ≥10%) was the most frequent physical abnormality, which occurred in 160 patients (13.9% overall and 12.7% versus 14.6% in without‐ and with‐chemo groups, respectively). No statistically relevant difference was observed between the safety profiles of the two patient subgroups without and with chemotherapy in the metastatic setting.
Discussion
The pivotal BOLERO‐2 trial showed that dual‐blockade based on the association of everolimus plus exemestane doubled the median progression‐free survival versus exemestane alone in patients with hormone receptor‐positive (HR+)/human epidermal growth receptor 2‐negative (HER2−) metastatic BC recurring/progressing on prior NSAIs. It also stated the importance of diligent monitoring, proactive communication, early detection, and implementation of appropriate AE management strategies [9] in those patients receiving the combination. In this scenario, the BALLET study has been initiated as the expanded access program focused on the safety profile of the combination. To our knowledge, it is the largest reported safety dataset on a patient population outside the restrictive criteria of a clinical trial of patients with HR+/HER2− BC progressing on prior NSAIs [10]; showing even the patients were more heavily pretreated, the safety profile of everolimus plus exemestane was consistent with the BOLERO‐2.
The current practice of the therapy of advanced/metastatic BC is based on sequential administration of different regimens that are considered as lines of treatment [2]. With the advent of new targeted agents, multiple lines of targeted therapy may be possible, and components of the combined regimens can overlap from one line to another. Noteworthy, a recent network meta‐analysis showed that combination as first‐ or second‐line therapy in HR+/HER− metastatic BC is more efficacious than several chemotherapy regimens that were reported in the literature along with favorable toxicities for the combination in most instances [11]. Thus, it is important to assess even the potential of cumulative and additive toxic effects among the drugs. With the increasingly widespread use of everolimus in the management of metastatic BC, more experience has to be accumulated on the safety in patient subgroups characterized by metastatic sites, prior and subsequent therapies including cytotoxic agents or radiation, and comorbidities.
The post hoc safety analysis, herein reported, revealed no statistically significant difference in the occurrence of AEs and SAEs between the two subgroups (namely, patients who never previously received chemotherapy in the metastatic setting and patients who previously received at least one chemotherapy treatment in the metastatic setting). The combination regimen of everolimus and exemestane, the only regimen currently registered with an mTOR inhibitor in this setting, represents a valid alternative to the harmful toxicity profile of cytotoxic chemotherapy, confirming what was previously reported [10], [11].
Furthermore, the present analyses relating to real‐world practice in Italy confirmed the side effects of the everolimus/exemestane combination are mainly confined to the toxicity profile of everolimus [2]. Data are consistent with overall European population, addressing the clinicians to pay particular attention to its administration in the first months [10].
The long‐term management of women with HR+ BC remains a challenge. Suboptimal response in some patients and relapse during or after therapy highlight the medical need of a better knowledge of the pathogenic mechanism along with a deeper understanding of the resistance to therapy itself. From the perspective of medical oncology, everolimus is a relatively new drug in the treatment of BC targeting the mTOR signaling involved in the endocrine resistance process. Although the data on safety and efficacy of everolimus are rapidly accumulating in patients with BC, the safety profile of everolimus is mostly consistent across all clinical trials [2], and there is an important need of continuous safety monitoring in the everyday clinical practice.
Stomatitis was the most frequent (51.3%) and relevant AE to be clinically focused on, in particular, with 53.1% in without‐ and 50.3% in with‐chemo group, without any significant difference. Prophylactic methodology might be a way to improve the stomatitis rate in these patients. In fact, recently published analysis (after the BALLET study was completed) suggests a correlation between stomatitis and efficacy of everolimus in patients with solid tumors, including metastatic BC, and a careful monitoring of patients is warranted [12]. The other commonly reported AE with everolimus is pneumonitis (interstitial lung disease), which occurred in 7.6% of the present study population (5.5% in without‐ and 8.7% in with‐chemo group, respectively) [13]. The reason for a lower incidence of pneumonitis compared with the previous reports [14] could be related to the increased awareness of the clinicians to possible initial symptoms, such as dyspnea, dry cough, fatigue, etc., and thus to their ability in an early diagnosis and management [13], [15]. Furthermore, the majority of pneumonitis and stomatitis in the present analysis were grade ≤2: 79.2% (468 out of 591) for stomatitis and 81.6 (71 out of 87) for pneumonitis, respectively.
Conclusion
The outcome of patients with advanced/metastatic BC is continuously improving because of the availability of new active agents. The combination of everolimus and exemestane in treating metastatic BC is a solid treatment option now largely used in Italy. This post hoc analysis of the BALLET study showed that previous chemotherapy did not affect the safety profile of the combination regimen based on everolimus and exemestane. Safety data on the Italian subset were representative of real‐world evidence and were consistent with both the overall European data and clinical trial results. However, new safety issues may emerge in long‐term survivors receiving the everolimus/exemestane therapy: it is important to continuously evaluate the safety data from everyday clinical practice.
Acknowledgments
We thank the patients who participated in the BALLET trial, study nurses, and clinical research associates from the individual trial centers who provided ongoing support; the investigators: Piazza Elena, A.O. Polo Univ. Luigi Sacco, Milano; Ceccherini Rita, Centro Oncologico Az. per i Serv. San. n. 1, Trieste; Farina Gabriella, Osp. Fatebenefratelli‐Oftalmico, Milano; Moroni Mauro, A. O. Ospedale S. Carlo Borromeo, Milano; Tondini Carlo Alberto, Ospedali Riuniti di Bergamo; Cinieri Saverio, Pres. Osp. A. Perrino ‐ ASL BR, Brindisi; Cairo Giuseppe, P.O. Vito Fazzi ASL Lecce, Lecce; Pisconti Salvatore, Pres. Osp. Centrale SS. Annunziata AUSL TA, Taranto; Palmiotti Gennaro, Osp. di Venere, Bari; Giotta Francesco, Istituto Tumori Giovanni Paolo II, Bari; Rizzi Anna, Fondazione Poliambulanza ‐ Istituto Ospedalieri di Brescia; Spada Massimiliano, Fondazione Istituto S. Raffaele Giuseppe Giglio Cefalù (PA); Pinotti Graziella, Pres. Osp. di Varese A.O. Osp. di Circolo, Varese; Caruso Francesco, Humanitas Centro Catanese di Oncologia, Catania; Mattioli Rodolfo, Az. Ospedali Riuniti Marche Nord, Fano (PU); Aieta Michele, IRCCS CROB, Rionero in Vulture (PZ); Graiff Claudio, Az. Sanit. dell'Alto Adige ‐ Comprensorio Sanit. di Bolzano; Verusio Claudio, P.O. di Saronno Az. Osp. Osp. di Circolo di Busto Arsizio, Saronno (VA); Aschele Carlo, Pres. Osp. S. Andrea ‐ Sede Felettino, La Spezia; Vicario Giovanni, P.O. di Castelfranco Veneto, Castelfranco Veneto (TV); Ferro Antonella, P.O. Osp. di Trento Az. Provinciale Servizi Sanitari, Trento; Alabiso Oscar, A.O. Maggiore della Carità, Novara; Pavesi Lorenzo, Istituto Scientifico di Pavia Fond. Salvatore Maugeri IRCCS, Pavia; Fava Sergio, Osp. Civile di Legnano, Milano; Pazzola Antonio, Pres. Osp. Osp. Civile SS. Annunziata, Sassari; Ruggeri Enzo Maria, Complesso Osp. di Belcolle, Viterbo; Tralongo Paolo, P.O. Osp. Umberto I, Siracusa; Giordano Monica, P.O. Osp. S. Anna, Como; D'Arco Alfonso Maria, P.O. Andrea Tortora, Nocera Inferiore (SA); Tinessa Vincenza, A.O. G.Rummo, Benevento; Gamucci Maria Teresa, P.O. Osp. S.S. Trinità di Sora ASL Frosinone, Sora (FR); Marchetti Paolo, A.O. Sant'Andrea, Roma; Cortesi Enrico, A.O. Policlinico Umberto I°, Roma; Portarena Ilaria, Fondazione Policlinico Tor Vergata, Roma; Danese Saverio, Osp. Ostetrico Ginecologico S. Anna, Torino; Amoroso Domenico, Osp. Versilia, Lido di Camaiore (LU); Cammilluzzi Eugenio, Osp. Sandro Pertini, Roma; Santoro Armando, IRCCS Istituto Clinico Humanitas, Rozzano (MI); Bretti Sergio, Osp. Civile di Ivrea; Farci Daniele, Osp. Oncologico A. Businco, Cagliari; Ianniello Giovanni Pietro, Az. Osp. Sant'Anna e San Sebastiano di Caserta; Palazzo Salvatore, P.O. Mariano Santo A.O. di Cosenza, Cosenza; Russo Antonio, Az. Osp.‐Univ. Policlinico P. Giaccone, Palermo; Rosti Giovanni, Osp. Regionale Ca' Foncello, Treviso; Marzano Nicola, P.O. S. Paolo, Bari; Maiello Evaristo, Osp. Casa Sollievo della Sofferenza, San Giovanni Rotondo (FG); Mairone Lorenza, Pres. Osp. Evangelico Valdese‐ASL 1 Torino, Torino; Maisano Roberto, Pres. Ospedali Riuniti A.O. Bianchi Melacrino Morelli, Reggio Calabria; Battaglia Caterina, P.O. Ciaccio‐De Lellis Az. Osp. Pugliese‐Ciaccio, Catanzaro; Grasso Donatella, IRCCS Policlinico San Matteo, Pavia; Gridelli Cesare, Az. Osp. S.G. Moscati, Avellino; Garrone Ornella A.O. S. Croce e Carle, Cuneo; Serravezza Giuseppe, P.O. F. Ferrari, Casarano (LE); Merlini Laura, P.O. San Bortolo, Vicenza; Ghiotto Cristina, Istituto Oncologico Veneto I.R.C.C.S., Padova; Saracchini Silvana, A.O. Santa Maria degli Angeli, Pordenone; Filippelli Gianfranco, P.O. San Francesco di Paola, Paola (CS); De Censi Andrea, Ospedale E.O. Ospedali Galliera, Genova; Ficorella Corrado, P.O. S. Salvatore, L'Aquila; Boni Corrado, Arcispedale S. Maria Nuova, Reggio Emilia; Di Stefano Pia, Presidio Ospedaliero Spirito Santo, Pescara; Donadio Michela, A.O. Città della Salute e della Scienza di Torino, Torino; Tagliaferri Pierosandro, Fond. per la Ricerca e la Cura dei Tumori “T. Campanella”, Catanzaro; Di Leo Angelo, P.O. Osp. Misericordia e Dolce, Prato; De Placido Sabino, Az. Osp. Univ. Policlinico Federico II, Napoli; Sarobba Giuseppina, Az. Osp.‐Univ. di Sassari, Sassari; Castiglione Federico, Osp. San Lazzaro ASL CN2, Alba (CN); Pisano Agata, Osp. S. Maria delle Grazie, Pozzuoli (NA); Fioretto Luisa, Stab. Osp. S. Maria Annunziata ‐ P.O. Sud‐Est, Bagno a Ripoli (FI); Soto Parra Hèctor, P.O. Gaspare Rodolico, Catania; Zampa Germano, Osp. P.T.P. Nuovo Regina Margherita, Roma; Iacono Carmelo, Presidio Ospedaliero Maria Paternò Arezzo ASP Ragusa, Ragusa; Montesarchio Vincenzo, P.O. D.Cotugno, Napoli; Barduagni Mario, P.O. L. Spolverini, Ariccia (RM); Benasso Marco, Osp. S. Paolo ASL 2 Savonese, Savona; Giustini Lucio, P.O. A. Murri ‐ Area Vasta n. 4 ASUR Marche, Femio (AP); Siena Salvatore, Az. Osp. Niguarda Ca' Granda, Milano; Barni Sandro, P.O. di Treviglio, Treviglio (BG); Bracarda Sergio, P.O. Area Aretina Nord S. Donato AUSL 8 AREZZO; Scinto Fedele, Ospedale Generale S.G. Calibita ‐ Fatebenefratelli, Roma; Visini Marilena, Osp. A. Manzoni, Lecco Milandri Carlo P.O. S. Giuseppe, Empoli (FI); Gianni Lorenzo, P.O. Osp. Infermi AUSL Rimini; Artale Salvatore, A.O. S. Antonio Abate, Gallarate (VA); Gianni Luca, Istituto S. Raffaele ‐ IRCCS, Milano; Ciardiello Fortunato, Az. Osp. Univ. Policl. Seconda Università di Napoli, Napoli; Tienghi Amelia, P.O. S. Maria delle Croci, Ravenna; Tortora Giampaolo, Ospedale Borgo Roma Az. Osp. Univ. Int. Verona, Verona; Brandes Alba, Osp. Bellaria, Bologna; Pasini Felice, Ospedale Civile ‐ Santa Maria della Misericordia, Rovigo; Adamo Vincenzo, Osp. Papardo A.O.R. Papardo‐Piemonte, Messina; Clerico Mario, Alberto Osp. degli Infermi di Biella; Cavazzini Giovanna, P.O. di Mantova A.O. C. Poma, Mantova; Gaion Fernando, Presidio Ospedaliero di Camposampiero, Camposampiero (PD); Scambia Giovanni, Policlinico Universitario A. Gemelli, Roma; Airoldi Mario, A.O. Città della Salute e della Scienza di Torino, Torino; Editorial support was provided by Content Ed Net, with the helpful contribution in drafting the test by Lisa Argnani, and was funded by Novartis Farma SpA (Origgio – IT). Statistical support was provided by Opis srl and was funded by Novartis Farma SpA Origgio (Origgio – IT). This study was sponsored by Novartis Pharmaceuticals Corporation. No grant number is applicable.
Contributor Information
Daniele Generali, Email: dgenerali@units.it.
Collaborators: Gabriella Mariani, Piazza Elena, Ceccherini Rita, Farina Gabriella, Moroni Mauro, Tondini Carlo Alberto, Cinieri Saverio, Cairo Giuseppe, Pisconti Salvatore, Palmiotti Gennaro, Giotta Francesco, Rizzi Anna, Spada Massimiliano, Pinotti Graziella, Caruso Francesco, Mattioli Rodolfo, Aieta Michele, Graiff Claudio, Verusio Claudio, Aschele Carlo, Sede Felettino, Vicario Giovanni, Ferro Antonella, Alabiso Oscar, Pavesi Lorenzo, Fava Sergio, Pazzola Antonio, Ruggeri Enzo Maria, Tralongo Paolo, Giordano Monica, D'Arco Alfonso Maria, Tinessa Vincenza, Gamucci Maria Teresa, Marchetti Paolo, Cortesi Enrico, Portarena Ilaria, Danese Saverio, Amoroso Domenico, Cammilluzzi Eugenio, Santoro Armando, Bretti Sergio, Farci Daniele, Ianniello Giovanni Pietro, Palazzo Salvatore, Russo Antonio, Rosti Giovanni, Marzano Nicola, Maiello Evaristo, Mairone Lorenza, Maisano Roberto, Battaglia Caterina, Grasso Donatella, Gridelli Cesare, Garrone Ornella, Serravezza Giuseppe, Merlini Laura, Ghiotto Cristina, Saracchini Silvana, Filippelli Gianfranco, De Censi Andrea, Ficorella Corrado, Boni Corrado, Di Stefano Pia, Donadio Michela, Tagliaferri Pierosandro, Di Leo Angelo, De Placido Sabino, Sarobba Giuseppina, Castiglione Federico, Pisano Agata, Fioretto Luisa, Soto Parra Hèctor, Zampa Germano, Iacono Carmelo, Montesarchio Vincenzo, Barduagni Mario, Benasso Marco, Giustini Lucio, Siena Salvatore, Barni Sandro, Bracarda Sergio, Scinto Fedele, Visini Marilena, Gianni Lorenzo, Artale Salvatore, Gianni Luca, Ciardiello Fortunato, Tienghi Amelia, Tortora Giampaolo, Brandes Alba, Pasini Felice, Adamo Vincenzo, Clerico Mario, Cavazzini Giovanna, Gaion Fernando, Scambia Giovanni, and Airoldi Mario
Author Contributions
Conception/Design: Daniele Generali, Gabriella Mariani
Provision of study material or patients: Daniele Generali, Filippo Montemurro, Roberto Bordonaro, Antonino Mafodda, Sante Romito, Andrea Michelotti, Pierluigi Piovano, Maria Teresa Ionta, Claudia Bighin, Donata Sartori, Antonio Frassoldati, Marina Elena Cazzaniga, Ferdinando Riccardi, Franco Testore, Patrizia Vici, Carlo Antonio Barone, Alessio Schirone, Federico Piacentini, Franco Nolè, Annamaria Molino, Luciano Latini, Edda Lucia Simoncini, Fausto Roila, Francesco Cognetti, Francesco Nuzzo, Jennifer Foglietta, Alessandro Marco Minisini, Gabriella Mariani
Collection and/or assembly of data: Daniele Generali, Filippo Montemurro, Roberto Bordonaro, Antonino Mafodda, Sante Romito, Andrea Michelotti, Pierluigi Piovano, Maria Teresa Ionta, Claudia Bighin, Donata Sartori, Antonio Frassoldati, Marina Elena Cazzaniga, Ferdinando Riccardi, Franco Testore, Patrizia Vici, Carlo Antonio Barone, Alessio Schirone, Federico Piacentini, Franco Nolè, Annamaria Molino, Luciano Latini, Edda Lucia Simoncini, Fausto Roila, Francesco Cognetti, Francesco Nuzzo, Jennifer Foglietta, Alessandro Marco Minisini, Gabriella Mariani
Data analysis and interpretation: Daniele Generali, Gabriella Mariani
Manuscript writing: Daniele Generali
Final approval of manuscript: Daniele Generali, Filippo Montemurro, Roberto Bordonaro, Antonino Mafodda, Sante Romito, Andrea Michelotti, Pierluigi Piovano, Maria Teresa Ionta, Claudia Bighin, Donata Sartori, Antonio Frassoldati, Marina Elena Cazzaniga, Ferdinando Riccardi, Franco Testore, Patrizia Vici, Carlo Antonio Barone, Alessio Schirone, Federico Piacentini, Franco Nolè, Annamaria Molino, Luciano Latini, Edda Lucia Simoncini, Fausto Roila, Francesco Cognetti, Francesco Nuzzo, Jennifer Foglietta, Alessandro Marco Minisini, Francesca Goffredo, Giuseppe Portera, Gilda Ascione, Gabriella Mariani
Disclosures
Daniele Generali: Novartis, Roche (RF), AstraZeneca, EISAI, Celgene (C/A); Filippo Montemurro: Roche S.p.A, Novartis S.p.A (H); Roberto Bordonaro: Novartis (C/A); Andrea Michelotti: Celgene, Roche, Novartis, AstraZeneca (C/A); Marina Elena Cazzaniga: Pierre Fabre, Novartis, Celgene (C/A); Federico Piacentini: Eisai, AstraZeneca, Roche (H); Francesco Nuzzo: Novartis, Pierre Fabre, GlaxoSmithKline (H); Alessandro Minisini: Novartis (C/A, RF); Gilda Ascione: Novartis (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Cardoso F, Costa A, Norton L et al. ESO‐ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Ann Oncol 2014;25:1871–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Študentová H, Vitásková D, Melichar B. Safety of mTOR inhibitors in breast cancer. Expert Opin Drug Saf 2016;15:1075–1085. [DOI] [PubMed] [Google Scholar]
- 3. Baselga J, Campone M, Piccart M et al. Everolimus in postmenopausal hormone‐receptor‐positive advanced breast cancer. N Engl J Med 2012;366:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Higgins MJ, Baselga J. Targeted therapies for breast cancer. J Clin Invest 2011;121:3797–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chia S, Gradishar W, Mauriac L et al. Double‐blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor‐positive, advanced breast cancer: Results from EFECT. J Clin Oncol 2008;26:1664–1670. [DOI] [PubMed] [Google Scholar]
- 6. Turner NC, Ro J, André F et al. Palbociclib in hormone‐receptor‐positive advanced breast cancer. N Engl J Med 2015;373:209–219. [DOI] [PubMed] [Google Scholar]
- 7. Gnant M. The role of mammalian target of rapamycin (mTOR) inhibition in the treatment of advanced breast cancer. Curr Oncol Rep 2013;15:14–23. [DOI] [PubMed] [Google Scholar]
- 8. Jerusalem G, Rorive A, Collignon J. Use of mTOR inhibitors in the treatment of breast cancer: An evaluation of factors that influence patient outcomes. Breast Cancer (Dove Med Press) 2014;6:43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baselga J, Campone M, Piccart M et al. Everolimus in postmenopausal hormone‐receptor‐positive advanced breast cancer. N Engl J Med 2012;366:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jerusalem G, Mariani G, Ciruelos EM et al. Safety of everolimus plus exemestane in patients with hormone‐receptor‐positive, HER2‐negative locally advanced or metastatic breast cancer progressing on prior non‐steroidal aromatase inhibitors: Primary results of a phase IIIb, open‐label, single‐arm, expanded‐access multicenter trial (BALLET). Ann Oncol 2016;27:1719–1725. [DOI] [PubMed] [Google Scholar]
- 11. Generali D, Venturini S, Rognoni C et al. A network meta‐analysis of everolimus plus exemestane versus chemotherapy in the first‐ and second‐line treatment of estrogen receptor‐positive metastatic breast cancer. Breast Cancer Res Treat 2015;152:95–117. [DOI] [PubMed] [Google Scholar]
- 12. Rugo HS, Hortobagyi GN, Yao J et al. Meta‐analysis of stomatitis in clinical studies of everolimus: Incidence and relationship with efficacy. Ann Oncol 2016;27:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Willemsen AE, Grutter JC, Gerritsen WR et al. mTOR inhibitor‐induced interstitial lung disease in cancer patients: Comprehensive review and a practical management algorithm. Int J Cancer 2016;138:2312–2321. [DOI] [PubMed] [Google Scholar]
- 14. Rugo HS, Pritchard KI, Gnant M et al. Incidence and time course of everolimus‐related adverse events in postmenopausal women with hormone receptor‐positive advanced breast cancer: Insights from BOLERO‐2. Ann Oncol 2014;25:808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Del Mastro L, Cazzaniga M, Solidoro P et al. Everolimus‐based therapy in patients with hormone receptor‐positive, HER2(‐) advanced breast cancer: Management considerations. Future Oncol 2015;11:2251–2254. [DOI] [PubMed] [Google Scholar]


