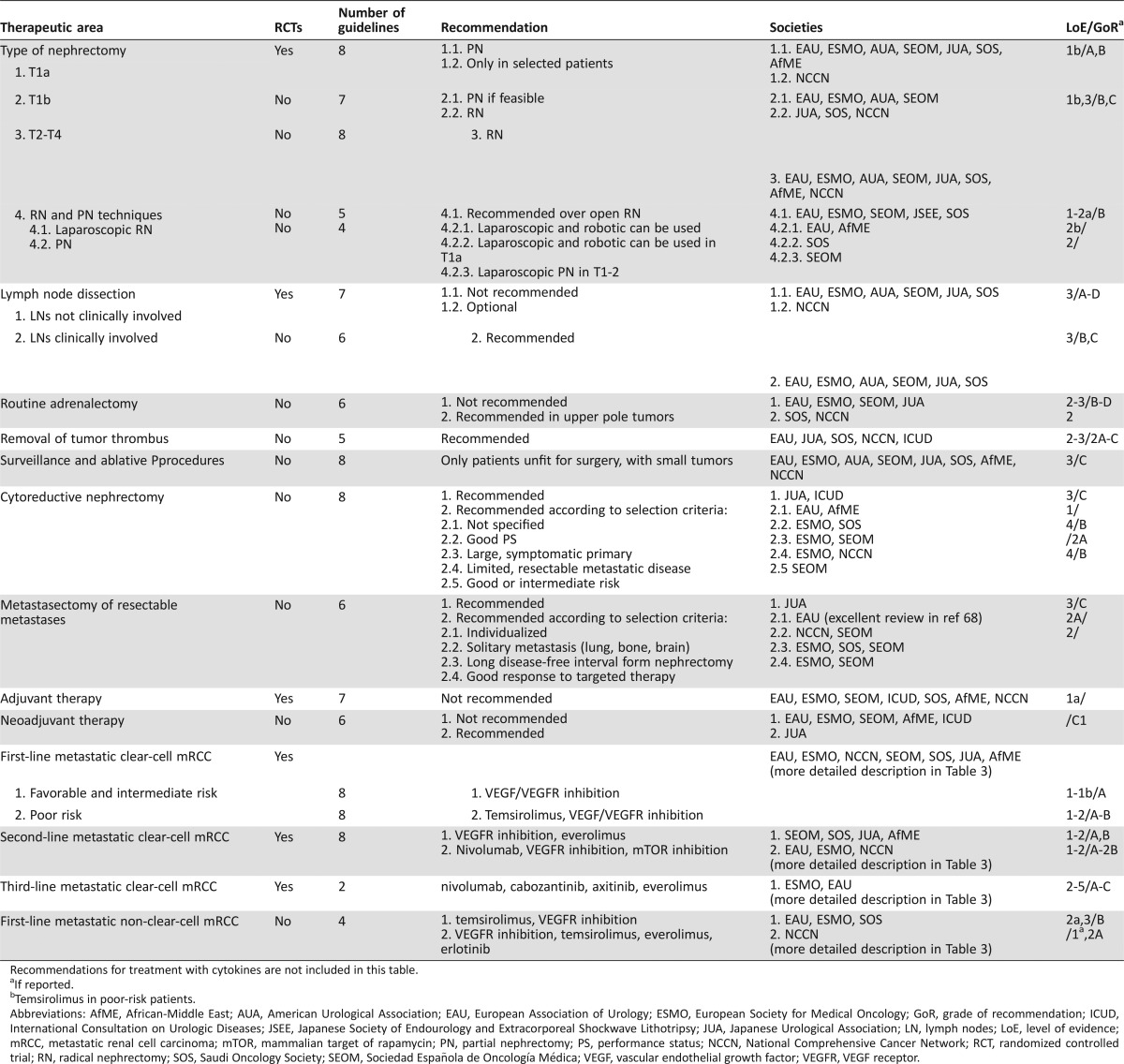
Table 2. Summary of recommendations included in the reviewed guidelines for the treatment of mRCC according to the availability of RCTs.
Recommendations for treatment with cytokines are not included in this table.
If reported.
bTemsirolimus in poor‐risk patients.
Abbreviations: AfME, African‐Middle East; AUA, American Urological Association; EAU, European Association of Urology; ESMO, European Society for Medical Oncology; GoR, grade of recommendation; ICUD, International Consultation on Urologic Diseases; JSEE, Japanese Society of Endourology and Extracorporeal Shockwave Lithotripsy; JUA, Japanese Urological Association; LN, lymph nodes; LoE, level of evidence; mRCC, metastatic renal cell carcinoma; mTOR, mammalian target of rapamycin; PN, partial nephrectomy; PS, performance status; NCCN, National Comprehensive Cancer Network; RCT, randomized controlled trial; RN, radical nephrectomy; SOS, Saudi Oncology Society; SEOM, Sociedad Española de Oncología Médica; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.