FIGURE 1:
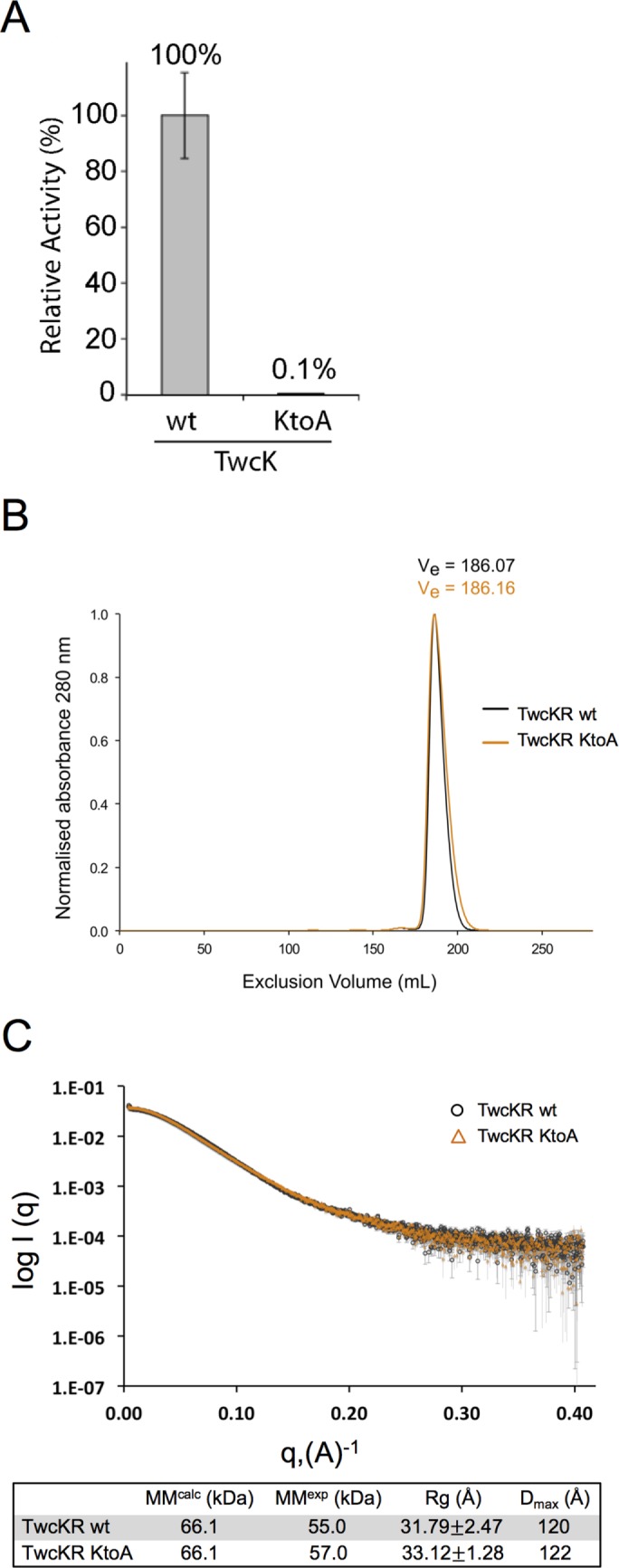
Mutation of a lysine involved in ATP coordination/phosphotransfer to alanine (K6290A) eliminates catalytic activity but has no effect on the overall structure of twitchin kinase in vitro. (A) The wild-type (wt) and the K6290A mutant forms of the catalytic domain of twitchin kinase (TwcK) were each expressed and purified from E. coli. The ability to phosphorylate a model peptide was measured with [γ-32P]ATP. As shown, the K6290A mutation almost entirely eliminates catalytic activity. (B) Size exclusion chromatograms of wt and mutant forms of a larger construct, TwcKR (Fn-NL-kin-CRD-Ig). TwcKR wt and TwcKR KtoA samples are coincident within experimental error, supporting the structural similarity of the samples. (C) SAXS analysis shows that the K6290A mutation does not affect the overall structure of the twitchin kinase region (TwcKR), so that the arrangement of assembled domains remains unaltered. Experimental scattering curves from TwcKR wt (black circle; capped error bars) and TwcKR K6290A (orange triangles; uncapped error bars) were scaled using DATADJUST (Petoukhov et al., 2012). The table below the graph lists molecular parameters calculated from the scattering data. MM, Rg, and Dmax denote molecular mass, radius of gyration, and maximal particle size, respectively. MMcalc is the theoretical MM of the constructs computed from primary sequence. Rg was calculated using AUTORG (Petoukhov et al., 2007).
