FIGURE 12:
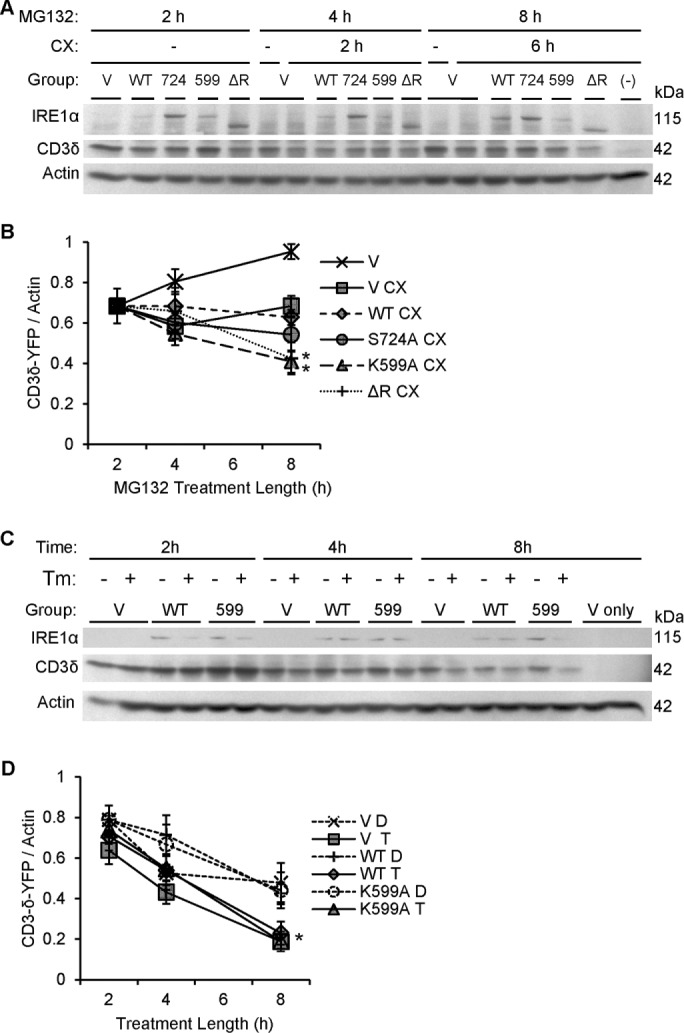
Dominant-negative IRE1α mutants (K599A and ΔR) accelerate degradation of CD3δ-YFP. (A) COS-1 cells cotransfected with FLAG-IRE1α (WT or mutants) and the ERAD reporter CD3δ-YFP were pretreated with MG132 (MG, 10 μM) to partially block the proteasome. At 2 h, vehicle (DMSO) or cycloheximide (CX, 25 μg/ml) was added. Lysates were immunoblotted after another 2 or 6 h, as indicated. Expression of IRE1α and CD3δ-YFP was confirmed using anti-FLAG and anti-GFP antibodies, respectively. Overexpression of IRE1α K599A or ΔR enhanced CD3δ-YFP degradation. The lane labeled “–” is a mock-transfected, cycloheximide-treated control sample, which confirms the CD3δ-YFP signal specificity. (B) Densitometric quantification showed that in the presence of cycloheximide, overexpression of IRE1α K599A or ΔR enhanced CD3δ-YFP degradation compared with vector. In addition, IRE1α K599A enhanced CD3δ-YFP degradation compared with WT (*p = 0.003 [IRE1α]; p = 0.0065 [CX]; p = 0.003 [IRE1α × time interaction; five experiments]. (C) COS-1 cells coexpressing IRE1α WT and CD3δ-YFP were cotreated with cycloheximide together with either vehicle (DMSO [D]) or tunicamycin (Tm or T, 5 μg/ml). Lysates were immunoblotted 2, 4, and 8 h posttreatment. The lanes labeled “V only” are without transfection of CD3δ-YFP. (D) Densitometric quantification shows that tunicamycin stimulated degradation of CD3δ-YFP in vector- and IRE1α-transfected cells. IRE1α did not modulate the tunicamycin-induced degradation of CD3δ-YFP, that is, overexpression of IRE1α WT did not potentiate tunicamycin-induced degradation of CD3δ-YFP, nor did IRE1α K599A exert an inhibitory effect (*p = 4.12 × 10−5 [Tm]; p = 3.70 × 10−15 [CX + time]; six experiments).
