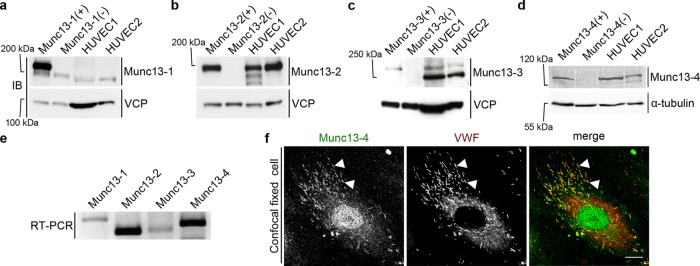
FIGURE 1:
Expression and localization of Munc13 proteins in human endothelial cells. (a–d) Western blot (IB) detection of Munc13-1 to -4 in two different HUVEC lysates. Included are positive (+) and negative controls (–), that is, mouse brain lysates (Munc13-1 to -3) or mouse lung lysate (Munc13-4) from wild-type or the respective knockout mouse. Calculated molecular masses are as follows: Munc13-1, 193 kDa; Munc13-2, 180 kDa; Munc13-3, 250 kDa (migrates at ∼280 kDa in SDS–PAGE (Varoqueaux et al., 2005); and Munc13-4, 123 kDa. Valosin-containing protein (VCP; 97 kDa) and α-tubulin (55 kDa) served as loading control. In a, the weaker band below Munc13-1 most likely corresponds to Munc13-2, which shows some cross-reactivity with the anti–Munc13-1 antibody (Cooper et al., 2012). The lower band in the HUVEC lysates in c likely stems from an unspecific antibody reaction, as it is not seen in the mouse lysates. (e) RT-PCR of HUVEC cDNA with primers amplifying specific sequences of all human Munc13 proteins (Munc13-1, 243 base pairs; Munc13-2, 159 base pairs; Munc13-3, 168 base pairs; Munc13-4, 201 base pairs). (f) Confocal section of fixed HUVECs labeled with anti–Munc13-4 and anti-VWF antibodies and Alexa Fluor 488– and Alexa Fluor 647–conjugated secondary antibodies. Arrowheads mark examples of colocalizations of Munc13-4 and VWF on WPBs. Scale bar, 10 µm.