Figure 2. The Combination of Ruxolitinib and Navitoclax Significantly Inhibited Tumor Growth and Resulted in Prolonged Survival of Tumor-bearing Mice.
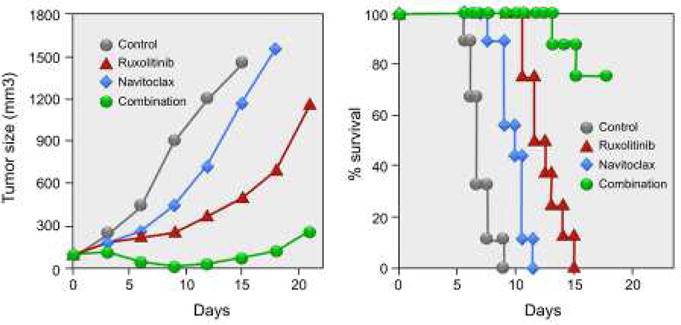
Combination therapy of ruxolitinib with navitoclax significantly inhibited ED(+)/IL-2 tumor growth in vivo. Female NSG mice were injected s.c. with 1 × 107 ED(+)/IL-2 cells. When the average tumor volume reached approximately 100 mm3, the tumor-bearing mice were divided into 4 groups (N = 8–9) with comparable average tumor volumes when the therapy was started. Ruxolitinib was continuously administered by a s.c. infusion pump at a dose of 50 mcg/kg/day for 14 days, and navitoclax was given orally at a dose of 40 mg/kg/day for 6 days. Average tumor volumes during the therapeutic course for reach group. Kaplan-Meier survival plot of the mice in the therapeutic study. Reproduced with modification from Figure 4B from the Proceedings of the National Academy of Sciences. Zhang M, Mathews Griner LA, Ju W, Duveau DY, Guha R, et al., 2015. Selective targeting of JAK/STAT signaling is potentiated by Bcl-xL blockade in IL-2-dependent adult T-cell leukemia. Proc Natl Acad Sci USA, volume 112 (40), pp. 12483.
Analysis of drug responses using in vitro culture in murine models were of value but may not reflect a facsimile of human disease states. Spontaneous proliferations of ex vivo PBMCs from patients with smoldering/chronic ATLL represented a model to assess the therapeutic potential of drug combinations in a human system. Proliferation of ATLLs in 6-day PBMC cultures assessed by 3H-thymidine uptake were determined from 5 patients with smoldering/chronic ATLL who were in the autocrine IL-2 dependent phase of their leukemia. Spontaneous proliferations of these PBMCs were partially inhibited by the addition of individual antibodies to IL-2 and to a lesser extent to IL-9 or IL-15. Profound inhibition of ex vivo proliferation was achieved by the simultaneous addition of antibodies against all 3 cytokines. Furthermore, addition of either ruxolitinib or navitoclax inhibited 6-day ex vivo proliferations of PBMCs from these patients. The combination of ruxolitinib with navitoclax provided enhanced inhibition (to 90%) of ex vivo proliferation of PBMCs from the 5 patients that was significantly greater than either drug alone (p < 0.01). Thus, the combination of ruxolitinib with navitoclax provided additive/synergistic activity with IL-2 dependent ATLL cell lines and in a mouse model of human IL-2 dependent ATLL as well as on ex vivo 6-day cultures of PBMCs from ATLL patients. These findings provide support for a therapeutic trial in patients with smoldering and chronic ATLL using a combination regimen that inhibits JAK1 and Bcl-xL. With further study it should be possible to provide effective multicomponent therapies for many T-cell malignancies that utilize as one of their elements an inhibitor of JAK1 or JAK3 kinase.
