Fig. 11.
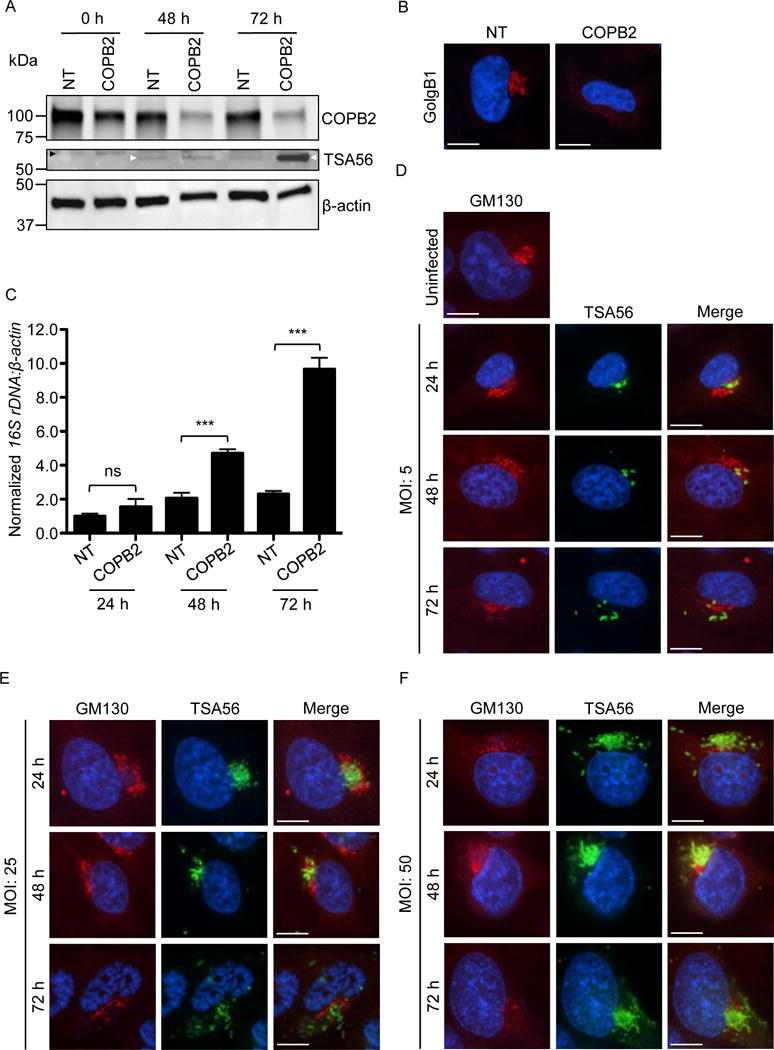
COPB2 knockdown increases Golgi instability and permissiveness to O. tsutsugamushi infection, and Orientia infection perturbs Golgi structure. (A–C) The Orientia load is higher and the Golgi is destabilized in cells in which COPB2 has been knocked down. (A) HeLa cells were treated with COPB2 or non-targeting (NT) siRNA for 48 h. The cells were infected with O. tsutsugamushi organisms (MOI of 10). Western blots of whole cell lysates generated at 0, 48, and 72 h post infection were screened with antibodies specific for COPB2, O. tsutsugamushi TSA56, and β-actin. White and black arrowheads denote the expected apparent molecular weights for TSA56 and a non-specifically recognized unknown HeLa cell protein, respectively. (B) At 48, NT and COPB2 siRNA treated uninfected HeLa cells were fixed, immunolabeled with GolgB1 antibody, stained with DAPI, and visualized by confocal microscopy. Results in A and B are representative of three experiments with similar results. (C) DNA isolated from HeLa cells that had been treated with NT or COPB2 siRNA for 48 h and infected with O. tsutsugaumushi for an additional 24, 48, or 72 h was analyzed by qPCR. Relative DNA levels of O. tsutsugamushi 16S rRNA gene were normalized to those of the human β-actin gene using the 2–ΔΔCT method. Results shown are the means ± standard deviation of triplicate samples and are representative of three independent experiments that yielded similar results. Statistically significant (***P < 0.001) values are indicated. ns, not significant. (D-F) O. tsutsugamushi infection promotes Golgi destabilization in bacterial dose- and time-dependent manners. HeLa cells infected with O. tsutsugamushi at MOIs of 5, 25, or 50 for 24, 48, or 72 h were fixed, immunolabeled with GM130 and TSA56 antibodies, stained with DAPI, and visualized by confocal microscopy. Results in D to F are representative of two experiments with similar results. Scale bars in B, D, E, and F are 10 μm.
