Fig. 7.
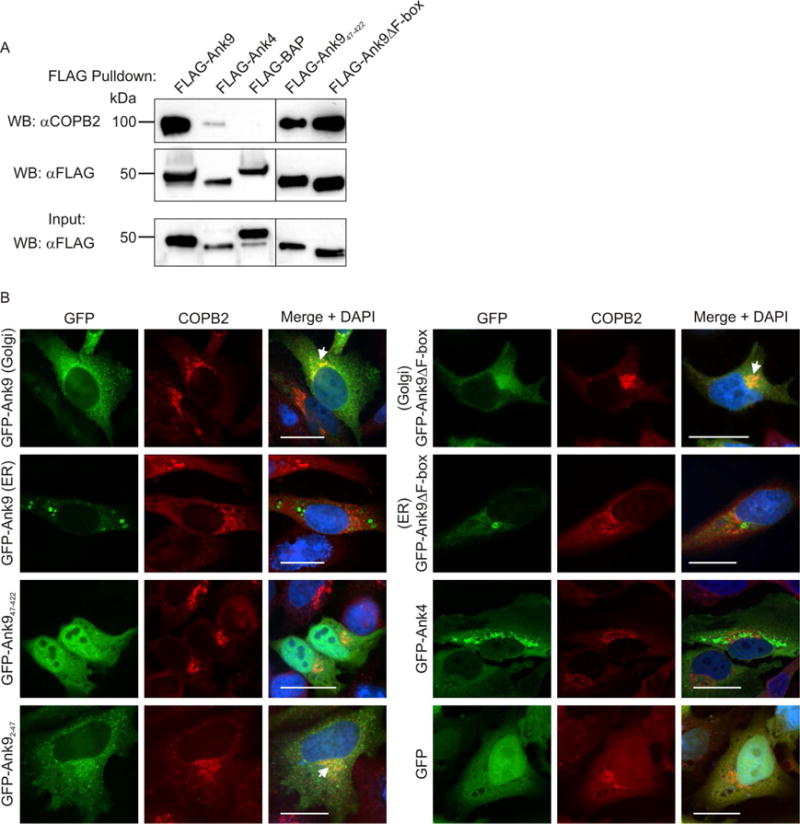
Endogenous COPB2 interacts with ectopically expressed Ank9 in a GLD- and F-box-independent manner and colocalizes with the Ank9 Golgi-associated but not ER-associated phenotype. (A) FLAG-Ank9 interacts with endogenous COPB2 in a GLD- and F-box-independent manner. Lysates of transfected HeLa cells expressing FLAG-tagged Ank9, Ank947–422 (lacks GLD), Ank9ΔF-box, Ank4, or BAP were incubated with FLAG antibody-conjugated agarose beads to immunoprecipitate FLAG-tagged proteins and co-precipitate interacting proteins. Resulting Western blots were probed with COPB2 antibody. Immunoprecipitation of FLAG-tagged proteins was verified by reprobing stripped blots with FLAG antibody. Expression of each FLAG-tagged protein of interest was confirmed by subjecting 3% of each input lysate to Western blotting using FLAG antibody. (B) GFP-Ank9 colocalizes with COPB2 in a GLD-dependent and F-box independent manner and only when Ank9 exhibits a Golgi-like localization pattern. HeLa cells expressing GFP tagged Ank9, Ank947–422, Ank92–47 (GLD only), Ank9ΔF-box, or the negative controls GFP-Ank4 or GFP alone were fixed, screened with COPB2 antibody, and examined by confocal microscopy. Representative fluorescence images of cells viewed for GFP, COPB2, and merged images plus DAPI are presented. Representative cells exhibiting GFP-Ank9 Golgi-associated (Golgi) and ER-associated (ER) subcellular localization patterns are denoted. White arrows designate representative points of GFP and COPB2 signal colocalization. Scale bars, 20 μm. Results shown are representative of three experiments with similar results.
