Abstract
Helicobacter pylori infection is associated with pronounced infiltration of granulocytes and lymphocytes into the gastric mucosa, resulting in active chronic gastritis that may develop into duodenal ulcer disease or gastric adenocarcinoma. Infiltrating T cells play a major role in the pathology of these diseases, but the signals involved in recruitment of T cells from blood to H. pylori-infected tissues are not well understood. We therefore examined H. pylori-induced T-cell transendothelial migration (TEM). The Transwell system, employing a monolayer of human umbilical vein endothelial cells, was used as a model to study TEM. H. pylori induced a significant T-cell migration, compared to spontaneous migration. CD4+ and CD8+ T cells migrated to the same extent in response to H. pylori, whereas there was significantly larger transmigration of memory T cells compared to naive T cells. Both H. pylori culture filtrate and urease induced migration, and the presence of the H. pylori cag pathogenicity island increased TEM. T-cell TEM was mediated by LFA-1-ICAM-1 interactions in accordance with an increased ICAM-1 expression on the endothelial cells after contact with H. pylori. Migrating T cells had increased expression of activation marker CD69 and chemokine receptors CXCR3, CCR4, and CCR9. Furthermore, T cells migrating in response to H. pylori secreted Th1 but not Th2 cytokines upon stimulation. In conclusion, our data indicate that live H. pylori and its secreted products contribute to T-cell recruitment to the gastric mucosa and that the responding T cells have an activated memory Th1 phenotype.
Helicobacter pylori is a gram-negative bacterium that colonizes the human stomach, as well as areas of gastric metaplasia in the duodenal bulb (30) H. pylori infection is associated with a pronounced infiltration of neutrophils and lymphocytes into the gastric mucosa, resulting in active chronic gastritis that may develop into duodenal ulcer disease or gastric adenocarcinoma (35). However, the mechanisms whereby H. pylori infection causes disease have as yet not been sufficiently elucidated. One factor strongly associated with H. pylori virulence and the development of duodenal ulcer disease is the cag (for cytotoxin-associated gene) pathogenicity island (PAI), which constitutes a gene cluster encoding a type IV secretion system (T4SS) (7).
It is generally held that specific subsets of infiltrating T cells also play an important role in controlling the outcome of infection via the cytokine response induced by the H. pylori infection. Several studies have shown that infection with this pathogen triggers a Th1-type immune response, i.e., effector CD4+ T cells producing gamma interferon (IFN-γ) but not interleukin-4 (IL-4), IL-5, or IL-13 in response to infection (2, 26, 38, 39). However, this response does not enable the immune system to clear the infection and may instead be detrimental to tissue integrity. Thus, the failure of the host response to clear the infection may in part be due to the type of immunity induced. However, even though T cells and their effector functions are of great importance for the pathology of H. pylori-associated diseases, the signals involved in the recruitment of T cells from the blood to H. pylori-infected tissue are not well characterized.
Transendothelial migration (TEM) of lymphocytes is essential in both steady-state lymphocyte recirculation and in recruitment to sites of inflammation. During TEM, chemokines presented on vascular endothelial cells mediate arrest of rolling lymphocytes by triggering integrin activation (6). Lymphocyte integrins, such as LFA-1 and VLA-4, then bind their respective counter receptors, ICAM-1 and VCAM-1, on the endothelial cells, thereby inducing firm arrest and diapedesis between endothelial cells. Furthermore, specific lymphocyte homing to gastrointestinal mucosal tissues is known to be dependent on expression of integrin α4β7 and chemokine receptor CCR9 (24). The continuous lymphocyte recruitment in chronic H. pylori infection is probably mediated by the combined actions of increased chemokine production and expression of adhesion molecules facilitating the interactions between lymphocytes and vascular endothelial cells (5, 43). In fact, it is known that H. pylori can induce the production of T-cell recruiting chemokines, such as IP-10 (gamma interferon-induced protein) and Mig (monokine induced by interferon gamma), in vivo (10), as well as increase the expression of the adhesion molecules VCAM-1 and ICAM-1, both on endothelial cells in vitro (21) and in H. pylori-infected individuals suffering from antral gastritis (18). In addition, recent studies by us and others indicate that α4β7-MADCAM-1 interactions are also necessary for lymphocyte homing to the gastric mucosa in H. pylori infection (28, 37).
Many studies have assessed the mechanism of T-cell TEM in response to chemokines such as I-TAC and RANTES (29, 40); however, bacteria might by themselves also induce TEM of T cells. This has currently only been shown for Borrelia burgdorferi (12), which preferentially induced migration of human CD8+ T cells. In order to elucidate the mechanisms behind H. pylori-induced T-cell recruitment, we examined TEM of human lymphocytes in response to the bacteria. We now show that H. pylori recruits both CD4+ and CD8+ T cells with a memory Th1 phenotype expressing CXCR3, CCR4, and CCR9 and that several H. pylori components contribute to this T-cell recruitment.
MATERIALS AND METHODS
Reagents.
Human umbilical vein endothelial cells (HUVEC), M200 medium, and a low-serum-growth supplement kit were purchased from Cascade Biologics, Inc. Dimethyl sulfoxide, polymyxin B, and jack bean (Canavalia ensiformis) urease were purchased from Sigma-Aldrich, Dorset, United Kingdom. Ficoll-Paque was retrieved from Pharmacia, Uppsala, Sweden. The Transwell filters were purchased from Costar, Babhoevedorp, The Netherlands, and fibronectin was purchased from Invitrogen, Taastrup, Denmark. Monoclonal blocking antibodies against LFA-1 (CD11a) and VCAM-1 (CD106) were purchased from R&D Systems, Abingdon, United Kingdom, as were the I-TAC, IP-10, and macrophage-derived chemokine (MDC) enzyme-linked immunosorbent assay (ELISA) kits. Isotype antibodies murine immunoglobulin G2a (mIgG2a) and mIgG1 were purchased from Dako Cytomation, Glostrup, Denmark, as were the antibodies used for the HUVEC stainings, ICAM-1 and VCAM-1. CD4- and CD8-positive isolation kits were purchased from Dynal ASA, Oslo, Norway. The gamma interferon (IFN-γ) and interleukin-13 (IL-13) ELISA kits and the CD4, CD8, CD45, CD45RA, CD45RO, CXCR3, CCR4, CCR9, CCR7 CXCR4, integrin B7, L-selectin, LFA-1, and VLA-4 antibodies were purchased from BD Pharmingen (BD), Stockholm, Sweden.
H. pylori strains and culture conditions.
H. pylori strains Hel 312 (cag PAI+, vacAs1/m1) and Hel 230 (cag PAI− vacA-subtype not tested by PCR) was isolated from the stomach of asymptomatic, adult, Swedish individuals, and Hel 305 (cag PAI+, vacAs1/m1) and Hel 333 (cag PAI−, vacAs2/m2) were isolated from the stomach of adult, Swedish duodenal-ulcer patients and characterized as previously described (15, 20). Mutants of different components of the H. pylori cag PAI T4SS were constructed from the cag PAI+ wild-type strain P1 by insertion of a chloramphenicol resistance gene cassette, as was the H. pylori ureA mutant. Correct insertion of the chloramphenicol resistance gene cassette was carefully checked by PCR. CagA expression was checked with monoclonal anti-CagA antibodies (Austral Biologicals), and no CagA phosphorylation was seen in anti-phosphotyrosine blots (antibody PY-99; Santa Cruz) (42). After isolation and initial culture, strains were stored in a freeze-drying medium containing 20% glycerol at −80°C. H. pylori bacteria were cultured on Columbia-Iso Agar plates for 3 days at 37°C in a microaerophilic milieu prior to stimulation. The bacteria were then resuspended in phosphate-buffered saline and adjusted to a final optical density at 600 nm (OD600) of 1.0 (Shimadzu UV-1201; Lambda Polynom, Stockholm, Sweden) corresponding to ca. 5 × 108 live H. pylori bacteria. The bacterial suspension was used directly for TEM experiments.
Production of H. pylori culture broth, lysate, and H. pylori components.
H. pylori culture broth was made by adding 2.5 ml of Hel 312 suspension to 48 ml of brucella broth medium, followed by incubation for 16 h at 37°C in a microaerophilic milieu.
H. pylori lysate was prepared as previously described by sonication and ultracentrifugation (14). The protein contents were determined by measuring the absorbance at 280 nm and calculating the amounts of protein in the samples. The conditioned broth and the lysate were sterile filtered and stored at −70°C until use. H. pylori urease was kindly provided by Ingrid Bölin (Department Medical Microbiology and Immunology, Göteborg, Sweden) and was purified from H. pylori strain E32 by combining the methods of Evans et al. (11) and Dunn et al. (9). The 26-kDa H. pylori protein, an AhpC homologue with alkyl hydroperoxide reductase activity (HP1563), was recombinantly produced in Escherichia coli (33) and kindly provided by the Genomic Therapeutic Company (Cambridge, Mass.). Possible H. pylori lipopolysaccharide (LPS) contaminations in the urease and 26-kDa preparations were inactivated by pretreatment with 10 μg of polymyxin B/ml for 1 h at room temperature. The actual LPS activities were determined by the Limulus endotoxin test to be 2.5 and 10 ng/ml, respectively. Inactivation of H. pylori strain Hel 312 was performed by adding formaldehyde to a final concentration of 10 mM to brucella broth-grown bacteria with an OD600 of 1.0. The bacterial suspension was then left on a shaker for 2 h at 37°C and at room temperature overnight. Formaldehyde-inactivated bacteria were then washed and stored at 4°C until use.
Subculture of endothelial cells.
HUVEC were obtained from the first passage and grown in M200 medium supplemented with the low serum growth supplement kit containing penicillin, streptomycin, amphotericin B, fetal bovine serum, hydrocortisone, human epidermal growth factor, basic fibroblast growth factor, and heparin in a humidified atmosphere of 5% CO2-95% air at 37°C. Medium was changed every 48 h. At passage 4, cells were frozen in fetal calf serum containing 10% dimethyl sulfoxide and stored in liquid nitrogen. HUVEC at passage 4 to 6 were used for all experiments.
Volunteers and isolation of PBMC.
The present study was approved by the Human Ethical Committee of Sahlgrenska University Hospital, and informed consent was obtained from all participating donors. Peripheral blood was collected from healthy Swedish H. pylori negative individuals (ages 25 to 40). Determination of H. pylori status was performed by serology as previously described (15) or with the EIA-G III ELISA (Orion Diagnostics). Peripheral blood mononuclear cells (PBMC) were isolated by density-gradient centrifugation on Ficoll-Paque. Isolated PBMC were then resuspended in M200 medium at a concentration of 107 cells/ml and kept on ice for a maximum of 1 h until use.
TEM of PBMC.
HUVEC were grown to confluence on collagen-coated Transwell filters (3 × 105/filter) in M200 complete medium supplemented with 10 μg of fibronectin/ml. Confluence was reached after 48 h and was confirmed by microscopic observation. Before TEM, the medium was changed, and the system was left to stabilize for 1 h. The migration-inducing stimuli were added basolaterally, and purified PBMC (1.5 × 106) were added apically. When whole bacteria were used, they were preincubated with the HUVEC for 4 h before addition of PBMC. All other stimulatory agents were added at the same time as the PBMC. Whole bacteria (OD600 = 1.0), formaldehyde-inactivated bacteria (OD600 = 1.0), and H. pylori culture filtrate were added at a volume of 100 μl, and H. pylori and jack bean urease, as well as the AhpC homologue, were added at a concentration of 10 μg/ml. All stimulations were run in duplicates. PBMC were then allowed to transmigrate for 16 h at 37°C. Thereafter, the medium in the lower chamber was collected, and migrating cells were resuspended in fluorescence-activated cell sorting (FACS) wash buffer (PBS containing 1.46 g of EDTA/liter, 2.5 g of serum albumin/liter, 0.2 g of NaN3/liter, and 2% AB+ human serum). The preincubation and transmigration times were optimized in pilot experiments.
In indicated experiments, neutralizing monoclonal antibodies against LFA-1 and VCAM-1 were used to block the respective adhesion molecules. Anti-LFA-1 (5 μg/ml) was incubated with PBMC for 30 min, and anti-VCAM-1 (20 μg/ml) was incubated with HUVEC for 1 h prior to migration. Purified mouse IgG2a and IgG1, respectively, served as isotype controls. Migrating lymphocytes were examined by using flow cytometry (FACSCalibur; BD) and identified based on forward- and side-scatter characteristics, as well as on the expression of CD45, and then counted by using TrueCount beads (BD). The expression of different lymphocyte surface markers on migrating cells was determined by using fluorescein isothiocyanate (FITC)-conjugated CD4, CD45RA, and CXCR3 antibodies and phycoerythrin (PE)-conjugated CD8, CD45RO, CCR4, CCR9, CCR7, and CXCR4 antibodies. The expression of adhesion molecules on the HUVEC cells after TEM was examined by using FITC-conjugated ICAM-1, VCAM-1, and MAdCAM-1. In addition, chemokine production in the apical Transwells after TEM toward H. pylori was also examined by using ELISA to detect the presence of I-TAC, IP-10, MDC, and RANTES.
Experiments were also performed to examine whether the bacterial preparations used for TEM were directly chemotactic for T cells in the absence of an endothelial monolayer. This was performed in the same manner as the TEM experiments but by using polycarbonate Transwell filters without HUVEC cells.
T-cell adhesion molecule expression after stimulation with H. pylori products.
A total of 5 × 105 PBMC were stimulated for 16 h with live H. pylori bacteria, H. pylori culture filtrate, or the 26-kDa protein in 96-well plates. Then, 30 μl of live bacteria and culture filtrate and 10 μg of the 26-kDa preparation/ml were added to each well. In addition, cells were also incubated with the apical supernatant obtained after 16 h of basolateral incubation of live H. pylori in Transwells with a HUVEC monolayer. T cells were then stained with PE-conjugated antibodies to VLA-4, integrin β7 and L-selectin, and FITC-conjugated anti-LFA-1 and analyzed by flow cytometry.
Cytokine production by migrating T cells.
IFN-γ and IL-13 production by T cells migrating in response to H. pylori was determined by allowing isolated CD4+ and CD8+ T cells to migrate toward 10 μg of H. pylori lysate/ml. Migrating cells were polyclonally stimulated with CD3 (25 ng/ml)- and CD28 (1 μg/ml)-specific antibodies in a 96-well plate previously coated with rat antibodies to mouse IgG (3 μg/ml). The cells remaining in the apical compartment, cells from the original cell suspension, as well as the original cells incubated with 10 μg of H. pylori lysate/ml for 16 h, were stimulated and analyzed in parallel. Stimulations were run in duplicates in Iscove complete medium with 105 cells per well. Supernatants were collected after 48 h and analyzed for their IFN-γ and IL-13 content by ELISA. All of the above cell preparations were also cultured without CD3/CD28 as a control.
Statistical analyses.
Statistical evaluation was performed by using the nonparametric Mann-Whitney test. P values of <0.05 were considered to be statistically significant.
RESULTS
H. pylori-induced TEM of lymphocytes.
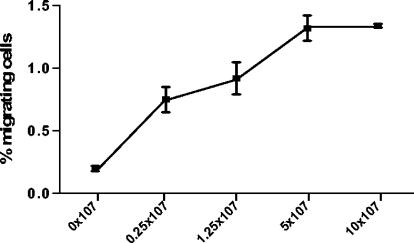
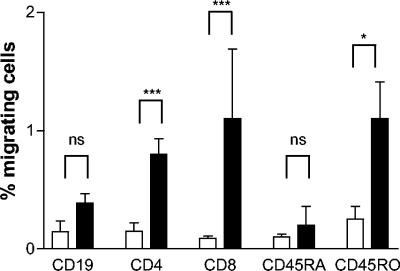
The Transwell system was used to investigate the possible TEM of human lymphocyte subsets in response to H. pylori. PBMC were added apically, and different bacterial stimuli were added to the basolateral side, where migrating cells were collected. Three well-characterized type I H. pylori strains (Hel 312, Hel 305, and P1), each carrying a functional cag PAI, were used (15, 20, 42), as well as two type II H. pylori strains (Hel 230 and Hel 333). Live H. pylori induced a consistent T-cell migration which was dose dependent and increased with higher numbers of bacteria in the lower chamber up to ca. 5 × 107 bacteria (Fig. 1). This concentration was used for all further studies. There was no difference in T-cell recruitment between type I and type II strains (data not shown), where both CD4+ and CD8+ cells showed a significantly (P < 0.0005) larger migration compared to the control. CD4+ and CD8+ cells migrated to the same extent, and the CD4/CD8 ratio was similar in the input cell suspension and the migrating cells. In contrast, there was no significant difference in spontaneous migration of B cells (CD19+) compared to B cells migrating in response to H. pylori (Fig. 2).
FIG. 1.
Dose-response curve of H. pylori-induced T-cell TEM. PBMC were incubated in Transwell chambers and allowed to migrate toward H. pylori strain Hel 312. The migrating cells were characterized and enumerated by flow cytometry. All samples were run in duplicates, and the number of migrating T cells is expressed as the percentage of the input cells. Squares indicate mean ± the standard error of the mean (SEM) of three different experiments.
FIG. 2.
H. pylori-induced TEM of different lymphocyte subsets. PBMC were incubated in Transwell chambers and allowed to migrate toward H. pylori strain Hel 312. The migrating cells were characterized and enumerated by using flow cytometry. All samples were run in duplicates, and the number of cells migrating spontaneously (□) or in response to H. pylori (▪) is expressed as the percentage of the input cells for the respective subsets. Bars indicate means ± the SEM of five different experiments. ✽, P < 0.05; ✽✽✽, P < 0.0005.
H. pylori components influencing T-cell TEM.
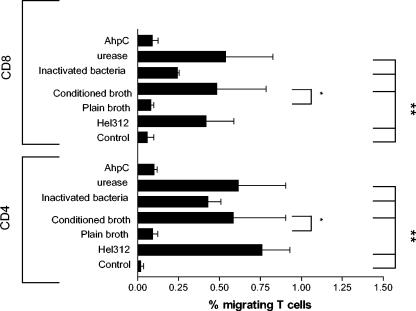
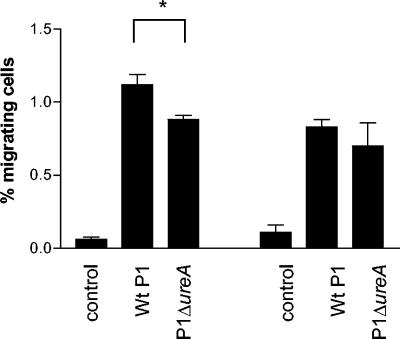
To further examine which H. pylori components induce T-cell migration, cells were allowed to migrate toward H. pylori culture filtrate, formaldehyde-inactivated H. pylori bacteria, urease, and the 26-kDa AhpC homologue. Both CD4+ and CD8+ T cells displayed a significantly (P < 0.05) larger migration toward culture filtrate than toward plain broth that had not been conditioned by H. pylori. In fact, migration toward H. pylori culture filtrate was as large as migration toward live H. pylori (Fig. 3). Both CD4+ and CD8+ T cells also showed a significantly (P < 0.005) larger TEM toward inactivated bacteria compared to the control, although this migration was lower than when live H. pylori was used. Moreover, 10 μg of H. pylori urease/ml had almost the same effect as whole H. pylori bacteria on T-cell migration, both with regard to the magnitude of the response and with regard to the cellular composition (Fig. 3). To confirm the effect of H. pylori urease on T-cell TEM, migration in response to a urease-producing strain (P1) and an isogenic mutant lacking the ureA subunit (P1Δ ureA) was performed. Indeed, CD4+ T cells showed a significantly lower migration toward the ureA mutant (P < 0.05). The same trend was seen for CD8+ T cells but due to individual variation the difference for CD8+ T cells did not reach statistical significance (Fig. 4). However, migration toward the ureA mutant was still significantly higher (P < 0.05) than that of the control. Furthermore, urease-induced T-cell TEM was H. pylori specific since jack bean urease did not induce an increased migration (data not shown). The urease preparation was treated with polymyxin B before TEM to inactivate possible LPS contamination. However, it has been argued that H. pylori LPS is resistant to polymyxin B treatment (27), so we tested the urease preparation for possible LPS activity before and after polymyxin treatment. Untreated urease contained 250 ng of LPS/ml, whereas polymyxin-treatment reduced the level of active LPS to 2.5 ng/ml. Since 10 μg of H. pylori LPS/ml did not induce TEM (data not shown), we conclude that urease is in fact one of the released H. pylori products involved in mediating T-cell recruitment. In contrast, the AhpC homologue induced no response above that of spontaneous migration (Fig. 3).
FIG. 3.
Identification of H. pylori components with effect on T-cell TEM. PBMC were allowed to migrate toward 5 × 107 live H. pylori bacteria, plain broth, H. pylori-conditioned broth, formaldehyde-inactivated bacteria, 10 μg of urease/ml, and 10 μg of AhpC homologue/ml. All samples were run in duplicates, and the migrating cells were characterized and enumerated by flow cytometry. The number of cells migrating is expressed as the percentage of the input cells. Bars indicate means ± the SEM of four to five different experiments. ✽, P < 0.05; ✽✽, P < 0.005.
FIG. 4.
Importance of H. pylori urease for T-cell TEM. PBMC were allowed to migrate toward a wild-type strain, P1, and an isogenic P1Δ ureA mutant. CD4+ (left side of figure) and CD8+ (right side of figure) migrating cells were characterized and enumerated by flow cytometry. All samples were run in duplicates, and the number of cells migrating is expressed as the percentage of the input cells. Bars indicate means ± the SEM of four different experiments. ✽, P < 0.05.
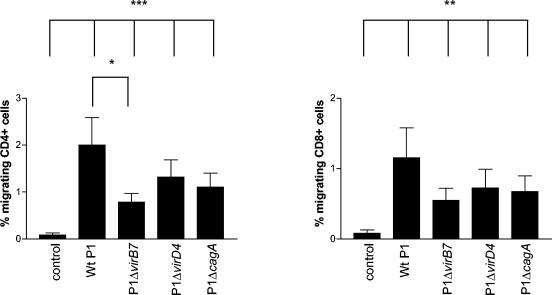
To determine whether the T4SS encoded by the cag PAI had any influence on T-cell TEM, migration in response to a cag PAI+ strain (P1) and three isogenic mutants with a nonfunctional T4SS (P1Δ virB7 and P1Δ virD4) or a deleted cagA gene (P1Δ cagA) was performed. CD4+ T cells showed a significantly lower migration toward the virB7 mutant (P < 0.05) and to a lesser extent also toward the virD4 and cagA mutants (Fig. 5). However, all cag PAI mutants induced a certain degree of TEM that was significantly higher (P < 0.005) than the control. CD8+ T cells also showed consistently lower migration toward the virB7 mutant, but the difference did not reach statistical significance (Fig. 5). Nevertheless, the composition of the migrating T cells was still the same. Taken together, these data indicate that several different H. pylori components, including the cag PAI, and factors released into the medium, such as urease, contribute to recruitment of T cells through the endothelial barrier.
FIG. 5.
Importance of the cag PAI for T-cell TEM. PBMC were allowed to migrate toward a wild-type strain, P1, and isogenic P1Δ virB7, P1Δ virD4, and P1Δ cagA mutants. CD4+ (left panel) and CD8+ (right panel) migrating cells were characterized and enumerated by using flow cytometry. All samples were run in duplicates, and the number of cells migrating is expressed as the percentage of the input cells. Bars indicate means ± the SEM of six different experiments. ✽, P < 0.05; ✽✽, P < 0.005; ✽✽✽, P < 0.0005.
Mechanisms of H. pylori-induced TEM.
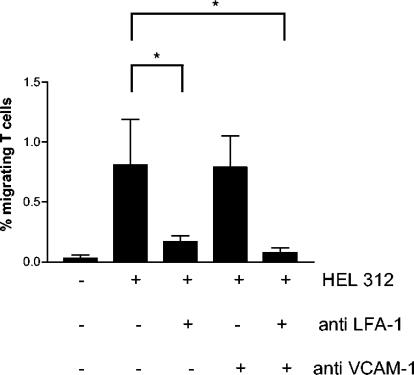
In order to determine whether the adhesion of T cells to the endothelial cells through LFA-1-ICAM-1 or VLA-4-VCAM-1 interactions was involved in H. pylori-induced recruitment of T cells, PBMC and HUVEC were incubated with antibodies to LFA-1 or VCAM-1, respectively. Indeed, blocking LFA-1 prior to migration toward H. pylori resulted in an inhibition of TEM by >80%. Preincubation of the HUVEC with an antibody against VCAM-1 showed no significant inhibition of migration. However, adding antibodies to both LFA-1 and VCAM-1 to the same Transwell resulted in a complete inhibition of TEM, almost to the level of spontaneous migration (Fig. 6). Incubation of PBMC and HUVEC with isotype control antibodies had no effect on T-cell TEM (data not shown).
FIG. 6.
Importance of LFA-1 and VCAM-1 for T-cell TEM. PBMC were allowed to migrate in response to H. pylori strain Hel 312 in the Transwell system with or without pretreatment of the lymphocytes with a monoclonal antibody to LFA-1 and/or pretreatment of the HUVEC monolayer with a monoclonal antibody to VCAM-1. The migrating cells were characterized and enumerated by flow cytometry. All samples were run in duplicates, and the number of cells migrating is expressed as the percentage of the input cells. Bars indicate means ± the SEM of four different experiments. ✽, P < 0.05.
To further determine the mechanisms underlying H. pylori-induced T-cell TEM, we examined migration of T cells toward the different H. pylori preparations used above in a Transwell system without a HUVEC monolayer. However, none of the H. pylori preparations were directly chemotactic for T cells in the absence of an endothelial monolayer (data not shown). We then examined the potential induction of T-cell adhesion molecules by in vitro stimulation of PBMC with the same H. pylori preparations or 100 μl of apical supernatant, obtained after 16 h basolateral incubation of live H. pylori in a Transwell with a HUVEC monolayer. Neither of these stimuli changed the level of expression of adhesion molecules LFA-1, VLA-4, integrin β7, or L-selectin on the T cells (data not shown).
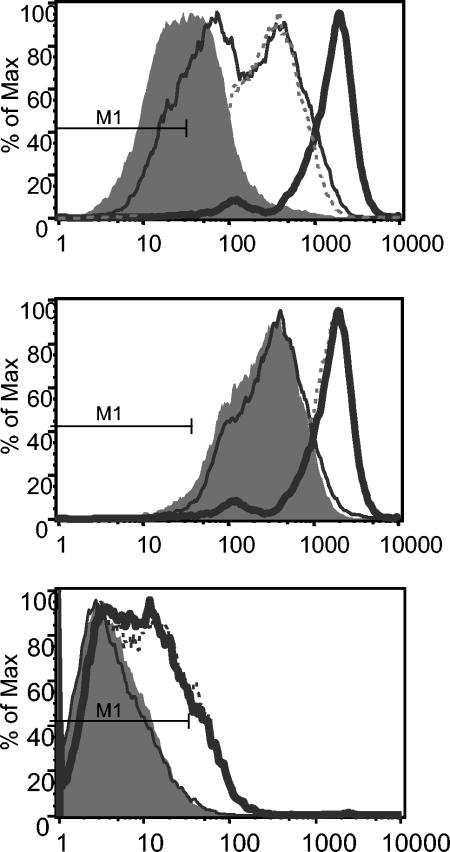
Since we showed that H. pylori-induced T-cell TEM is dependent on LFA-1-ICAM-1 interactions, we investigated whether there were any changes in adhesion molecule expression on the HUVEC after basolateral incubation with H. pylori in the Transwell system. Incubation with the bacteria for 16 h induced an increased expression of ICAM-1 on HUVEC (Fig. 7, top panel). An almost identical ICAM-1 expression was also induced by allowing spontaneous TEM by PBMC (i.e., without any H. pylori stimulation). The addition of T cells to H. pylori-stimulated HUVEC further augmented ICAM-1 expression, indicating contribution of both H. pylori and PBMC to the detected increase in ICAM-1 (median fluorescence fold increase of 5.4; P = 0.002) (Fig. 7, top panel). A similar pattern was also seen for VCAM-1 expression (median fluorescence fold increase of 1.3; P = 0.02) (data not shown). A similar increase in ICAM-1 and VCAM-1 expression was also seen after H. pylori urease-induced TEM (P = 0.02 and P = 0.04, respectively) but not after migration toward the H. pylori 26-kDa protein, a poor inducer of T-cell TEM (Fig. 7, middle and bottom panels). Furthermore, no expression of MAdCAM-1 could be detected on HUVEC after H. pylori incubation.
FIG. 7.
Adhesion molecule expression by HUVEC after H. pylori-induced TEM. PBMC were allowed to migrate toward H. pylori strain Hel 312, urease, or the 26-kDa protein in the Transwell system, and investigation of ICAM-1 (top and middle panels) and VCAM-1 (bottom panel) expression on HUVEC after TEM was performed by using flow cytometry. The top panel shows ICAM-1 expression with or without PBMC and H. pylori added. The filled histogram shows ICAM-1 expression on untreated HUVEC, the thin solid line shows ICAM-1 expression after addition of H. pylori alone, the dashed line shows ICAM-1 expression after addition of PBMC alone, and the bold solid line shows ICAM-1 after the addition of both H. pylori and PBMC for 16 h, allowing TEM. The middle and bottom panels show ICAM-1 and VCAM-1 expression on HUVEC after transmigration of PBMC toward medium (filled histogram), Hel 312 (bold solid line), urease (dashed line), or 26-kDa protein (thin solid line). M1 indicates the fluorescence of the isotype control in all histograms.
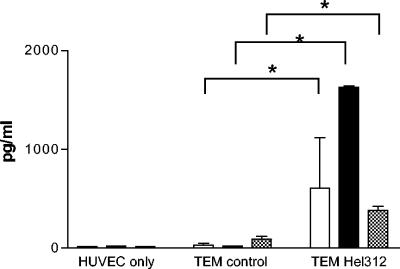
In parallel, investigation of the chemokines produced in the apical compartment during H. pylori-induced TEM was performed. There was a significant increase in the T-cell recruiting chemokines IP-10 (CXCL10) and I-TAC (CXCL11), as well as MDC (CXCL22), in the apical compartment of Transwells with H. pylori compared to control (Fig. 8). In contrast, RANTES was present at equal levels regardless of H. pylori stimulation (data not shown).
FIG. 8.
Chemokine content in the apical Transwell during H. pylori-induced TEM. PBMC were allowed to migrate spontaneously (TEM control) or toward H. pylori strain Hel 312 (TEM Hel 312) in the Transwell system for 16 h. After TEM, medium was collected from the apical compartment, and I-TAC (□), IP-10 (▪), and MDC (▩) production was measured by ELISA. All samples were run in duplicates, and the bars indicate means ± the SEM of three to five different experiments. ✽, P < 0.05.
Characterization of migrating T cells.
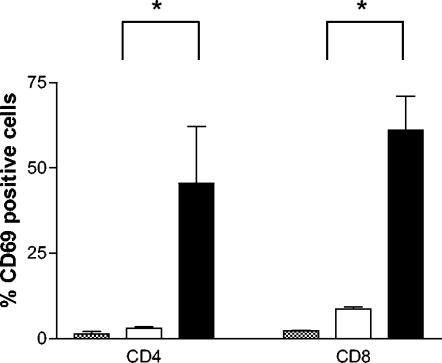
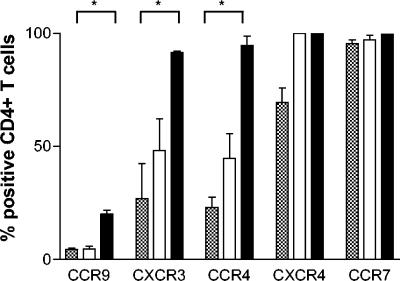
To characterize the specific subsets of T cells that migrate in response to H. pylori, the expression of selected surface markers was examined by using flow cytometry. Even though CD4+ and CD8+ T cells migrated to the same extent toward H. pylori, there was a significantly (P < 0.05) larger migration of putative memory cells (CD45RO+) compared to naive cells (CD45RA+), the latter cells migrating only at the same level as unstimulated control cells (Fig. 1). The early activation marker CD69 was expressed on approximately half of the CD4+ and CD8+ T cells that migrated toward H. pylori. In contrast, only a few of the T cells that migrated spontaneously or those in the original cell suspension expressed CD69 (Fig. 9). Next, the expression of chemokine receptors on migrating T cells was examined. H. pylori-responsive CD4+ T cells had a significantly higher expression of the “inflammatory” chemokine receptors CXCR3 and CCR4, which are often coexpressed on activated Th1 cells (23), and the gut homing chemokine receptor CCR9, than CD4+ T cells in the original cell suspension or the spontaneously migrating CD4+ T cells (Fig. 10). In contrast, the “homeostatic” chemokine receptors CXCR4 and CCR7 were present on all migrating cells, regardless of H. pylori stimulation. Incubation of peripheral blood T cells with H. pylori bacteria for 16 h did not induce increased CD69 or chemokine receptor expression.
FIG. 9.
CD69 expression by T cells migrating in response to H. pylori. PBMC were allowed to migrate toward H. pylori strain Hel 312 in the Transwell system, and the expression of CD69 on migrating cells was analyzed by flow cytometry. The expression of CD69 on T cells migrating spontaneously (□) or in response to H. pylori (▪) is compared to CD69 expression in the original cell suspension (░⃞). All samples were run in duplicates, and the bars indicate means ± the SEM of five different experiments. ✽, P < 0.05.
FIG. 10.
Chemokine receptor expression by T cells migrating in response to H. pylori. PBMC were allowed to migrate toward H. pylori strain Hel 312 in the Transwell system, and the expression of chemokine receptors on CD4+ T cells was analyzed by flow cytometry. The expression of the respective chemokine receptors on T cells migrating spontaneously (□) or in response to H. pylori (▪) is compared to chemokine receptor expression in the original cell suspension (░⃞). All samples were run in duplicates and the bars indicate mean ± the SEM of five different experiments. ✽, P < 0.05.
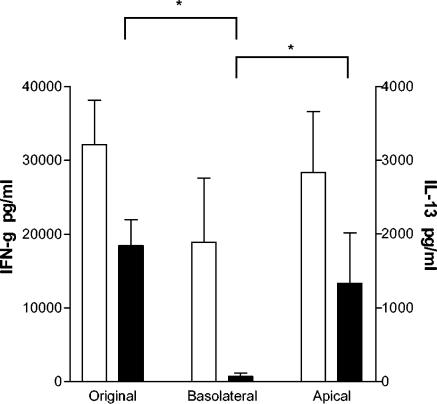
Since migrating T cells expressed Th1-associated chemokine receptors, we tried to further determine whether H. pylori recruits cells with a Th1 phenotype by looking at Th1 and Th2 cytokine secretion (IFN-γ and IL-13, respectively) by cells migrating in response to H. pylori lysate. Since monocytes have been shown to produce the Th1 promoting cytokine IL-12 upon contact with H. pylori (8), these experiments were performed with purified CD4+ and CD8+ T cells. Moreover, T cells were allowed to migrate toward H. pylori lysate instead of live bacteria to avoid live H. pylori in our stimulation assay. Cells migrating in response to H. pylori lysate produced high levels of IFN-γ after 48 h of stimulation (Fig. 11), as did cells from the original cell suspension and the remaining nonmigrating cells in the apical compartment. However, cells migrating in response to H. pylori lysate did not produce any IL-13, whereas cells from the original cell suspension and nonmigrating cells readily did (Fig. 11). Incubation of T cells with H. pylori lysate for 16 h before stimulation, to mimic the environment of migrated cells in the basolateral compartment, did not influence either IFN-γ or IL-13 secretion (data not shown). These data show that H. pylori do not recruit any Th2 cells but only T cells with a Th1 phenotype.
FIG. 11.
Cytokine production by cells migrating in response to H. pylori lysate. PBMC were allowed to migrate toward H. pylori lysate (10 μg/ml) in the Transwell system and thereafter stimulated with CD3/CD28 antibodies. Cells from the apical compartment and cells from the original cell suspension were stimulated in parallel. Supernatants were collected after 48 h, and IFN-γ (□) and IL-13 (▪) production was measured by ELISA. All samples were run in duplicates, and the bars indicate means ± the SEM of three different experiments. ✽, P < 0.05 (as determined by Mann-Whitney one-tailed nonparametric test).
DISCUSSION
In this study we investigated the TEM of different human lymphocyte subsets in response to H. pylori, based on the assumption that recruitment of specific subsets of T cells to the gastric mucosa in H. pylori infection plays an important role in the outcome of infection. We were able to show that substantial numbers of T cells traverse the endothelium in response to H. pylori, whereas this was not the case for B cells. In accordance with previous studies on T-cell migration in response to chemokines (29, 40), both CD4+ and CD8+ T cells migrated to the same extent and indeed, increased numbers of both CD4+ and CD8+ T cells have been shown to be present in H. pylori-infected gastric tissue (2, 17). This is in contrast to the only previous study on T-cell TEM induced by bacteria showing a greater migration of CD8+ T cells than CD4+ T cells (12), indicating existence of specific patterns of bacterium-induced T-cell recruitment. Moreover, in accordance with previous in vitro studies (4, 36), we showed enrichment of CD45RO+ cells in the migrating T-cell population, indicating that memory cells express specific homing markers that naive cells do not.
Recent studies in our group have shown that neutrophils migrate toward H. pylori conditioned medium as well as toward live bacteria (M. Brisslert et al., unpublished data). We found the same to be true for T cells, a finding implying that released bacterial products are involved in mediating this recruitment. Urease is one such product that has previously been shown to stimulate macrophages (16) and to induce neutrophil TEM (Brisslert et al., unpublished), and our data suggest that H. pylori urease is important also in recruiting T cells to H. pylori-infected mucosal tissue. In vitro studies have reported that H. pylori proteins can be actively transported through gastric epithelial monolayers (31) and that H. pylori bacteria and bacterial products can disrupt epithelial barriers (45, 46). Historically, H. pylori has been considered a noninvasive bacteria (30), but in recent years electron microscopy studies have demonstrated H. pylori in the cytoplasm of gastric epithelial cells (25, 34) or even in the gastric lamina propria (22). Based on these observations, we hypothesize that several H. pylori components, such as urease, are located in the lamina propria during infection and that these components probably work in parallel to induce recruitment of T cells through the endothelial barrier.
Another such factor is probably the cag PAI gene cluster. The observation that cag PAI+ type 1 strains and cag PAI− type 2 strains showed no difference in their abilities to induce T-cell migration probably reflects the multifactorial nature of the H. pylori-induced TEM, an effect that is overcome when isogenic strains are used. However, translocation of the CagA protein is probably not the major stimulus for T-cell migration, since deletion of the virD4 gene, involved in CagA translocation, or of the cagA gene itself did not affect T-cell recruitment to the same extent as the virB7 mutant. These data are in accordance with recent findings from our group and others showing that virB mutants do not induce increased adhesion molecule expression or IL-8 secretion by endothelial and gastric epithelial cells to the same extent as wild-type bacteria or virD4 and vir cagA mutants do (42; our unpublished data), indicating that an indirect effect via endothelial cells might explain the reduced T-cell TEM induced by the virB mutants.
In order to further elucidate the mechanisms involved in H. pylori-induced TEM, the importance of selected molecules known to be involved in T-cell diapedesis was investigated. H. pylori induced increased expression of both ICAM-1 and VCAM-1 on HUVEC after TEM. Blocking experiments showed that T-cell migration toward H. pylori is mostly LFA-1-ICAM-1 dependent, as indicated also by the larger increase in ICAM-1 compared to VCAM-1 expression after H. pylori stimulation. The fact that VCAM-1 also was significantly upregulated might suggest that ICAM-1 and VCAM-1 have different functions in the T-cell migration process, as previously shown by Oppenheimer-Marks et al. (32). The adhesion of T cells to activated endothelia was shown to be VCAM-1-VLA-4 dependent, whereas the actual TEM was ICAM-1-LFA-1 dependent. ICAM-1 and VCAM-1 expression correlated with the induction of T-cell TEM, since live bacteria and urease but not the poor T-cell TEM inducer (26 kDa) increased the expression. Taken together, with the lack of T-cell migration without endothelial cells, our data lead to the conclusion that H. pylori or purified bacterial components recruit T cells via effects on the endothelium. Since the addition of T cells to H. pylori-stimulated HUVEC further augmented the expression of ICAM-1 and VCAM-1, not only the bacteria but also the PBMC population contribute to the final endothelial activation.
Tissue-specific lymphocyte migration to gastrointestinal tissues is dependent on the expression of integrin α4β7, which binds to MAdCAM-1 on endothelial cells in the gastrointestinal tract and mesenteric lymph nodes (3). Unstimulated HUVEC do not express MAdCAM-1, and we could not detect any expression of MAdCAM-1 after stimulation with live H. pylori (data not shown); hence, T-cell TEM in our system is more likely to be mediated through binding of T cells to ICAM-1 and VCAM-1. This is in accordance with a study by Higuchi et al. (19) showing ICAM-1 to be the most predominant among the adhesion molecules expressed in chronic H. pylori infection. Furthermore, we have also shown that MAdCAM-1 expression in the human gastric mucosa does not change during H. pylori infection (37). Differential expression of chemokines and chemokine receptors are also crucial for tissue-specific T-cell recruitment. For example, CCR9, whose ligand TECK is produced in the small intestine, is preferentially expressed on lymphocytes carrying the mucosal homing receptor integrin α4β7. In addition, Th1 and Th2 cells have been suggested to differ in their response to chemokines, which may explain the preferential accumulation of Th1 cells in many acute and chronic inflammatory diseases (6, 41). Cells migrating toward H. pylori were highly enriched in T cells expressing the chemokine receptors CXCR3, CCR4, and CCR9. CCR4 was generally held to be expressed on Th2 cells, but CXCR3 and CCR4 are also coexpressed on activated Th1 cells, whereas CXCR3 is never expressed on Th2 cells (1, 23). In agreement, the CXCR3 ligands, IP-10 and Mig, have been shown to be prominent in H. pylori-induced gastritis and seem to colocalize with infiltrating T cells (10). Moreover, the CXCR3 ligands IP-10 and I-TAC, as well as the CCR4 ligand MDC, were present in the apical compartment after H. pylori-induced TEM. Endothelial cells are known producers of chemokines, but since H. pylori culture with HUVEC cells alone did not induce any production of the studied chemokines, we suggest that H. pylori products activate the endothelium, which in turn activates the lymphocytes or the monocytes to either directly secrete chemokines or act back on the endothelium that then produces the chemokines, supporting the subsequent TEM of CXCR3+ and CCR4+ T cells.
Moreover, the majority of both CD4+ and CD8+ migrating T cells expressed the activation marker CD69. Since the transmigration process per se (spontaneous migration) or mere contact with H. pylori bacteria did not induce either CD69 expression or chemokine receptor expression, we conclude that H. pylori selectively recruits newly activated T cells with a Th1 phenotype. Indeed, CD69+ T cells are abundant in the H. pylori-infected gastric mucosa (44), and Th1 cells are known to dominate in H. pylori-induced gastritis (2).
In accordance with the expression of Th1 associated chemokine receptors on migrating T cells, cells migrating toward H. pylori lysate did not secrete the Th2 cytokine IL-13 but instead had the potential to secrete the Th1 cytokine IFN-γ, further strengthening our conclusion that H. pylori specifically recruits newly activated T cells with a Th1 phenotype. This is in contrast to a recent report by Gergel and Furie (13) indicating that T cells migrating toward the bacteria B. burgdorferi had elevated levels of IFN-γ production but similar levels of IL-4 production compared to the initial cell population.
In the present study, we show for the first time that H. pylori recruits T cells with the same phenotype as that found in the gastric mucosa in vivo, namely, memory CD4+ and CD8+ T cells with a Th1 phenotype. Furthermore, these T cells express the activation marker CD69 and migrate in an LFA-1-dependent fashion. Our findings suggest that live H. pylori and its secreted products, such as urease, contribute to recruitment of Th1 cells to the gastric mucosa, via effects on the endothelial cells, a feature that may have a profound effect on the outcome of the infection.
Acknowledgments
We are grateful to the volunteers participating in this study, as well as to Camilla Johansson and Kerstin Andersson at the Department of Medical Microbiology and Immunology, Göteborg University, Gothenburg, Sweden, for their valuable help.
This study was supported by the Swedish Science Council (06X-13428), Claes Groschinsky's Foundation, Magnus Bergwall's Foundation, Nanne Svart's Foundation, and Helge Axson Johnson's Foundation.
Editor: J. T. Barbieri
REFERENCES
- 1.Andrew, D. P., N. Ruffing, C. H. Kim, W. Miao, H. Heath, Y. Li, K. Murphy, J. J. Campbell, E. C. Butcher, and L. Wu. 2001. C-C chemokine receptor 4 expression defines a major subset of circulating nonintestinal memory T cells of both Th1 and Th2 potential. J. Immunol. 166:103-111. [DOI] [PubMed] [Google Scholar]
- 2.Bamford, K. B., X. Fan, S. E. Crowe, J. F. Leary, W. K. Gourley, G. K. Luthra, E. G. Brooks, D. Y. Graham, V. E. Reyes, and P. B. Ernst. 1998. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology 114:482-492. [DOI] [PubMed] [Google Scholar]
- 3.Berlin, C., E. L. Berg, M. J. Briskin, D. P. Andrew, P. J. Kilshaw, B. Holzmann, I. L. Weissman, A. Hamann, and E. C. Butcher. 1993. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74:185-186. [DOI] [PubMed] [Google Scholar]
- 4.Brezinschek, R. I., P. E. Lipsky, P. Galea, R. Vita, and N. Oppenheimer-Marks. 1995. Phenotypic characterization of CD4+ T cells that exhibit a transendothelial migratory capacity. J. Immunol. 154:3062-3077. [PubMed] [Google Scholar]
- 5.Butcher, E. C., and L. J. Picker. 1996. Lymphocyte homing and homeostasis. Science 272:60-66. [DOI] [PubMed] [Google Scholar]
- 6.Campbell, J. J., and E. C. Butcher. 2000. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr. Opin. Immunol. 12:336-341. [DOI] [PubMed] [Google Scholar]
- 7.Covacci, A., J. L. Telford, G. Del Giudice, J. Parsonnet, and R. Rappuoli. 1999. Helicobacter pylori virulence and genetic geography. Science 284:1328-1333. [DOI] [PubMed] [Google Scholar]
- 8.de Jonge, R., J. G. Kusters, M. S. Timmer, V. Gimmel, B. J. Appelmelk, S. Bereswill, A. H. van Vliet, S. G. Meuwissen, M. Kist, C. M. Vandenbroucke-Grauls, and E. J. Kuipers. 2001. The role of Helicobacter pylori virulence factors in interleukin production by monocytic cells. FEMS Microbiol. Lett. 196:235-238. [DOI] [PubMed] [Google Scholar]
- 9.Dunn, B. E., G. P. Campbell, G. I. Perez-Perez, and M. J. Blaser. 1990. Purification and characterization of urease from Helicobacter pylori. J. Biol. Chem. 265:9464-9469. [PubMed] [Google Scholar]
- 10.Eck, M., B. Schmausser, K. Scheller, A. Toksoy, M. Kraus, T. Menzel, H. K. Muller-Hermelink, and R. Gillitzer. 2000. CXC chemokines Gro(alpha)/IL-8 and IP-10/MIG in Helicobacter pylori gastritis. Clin. Exp. Immunol. 122:192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans, D. J., Jr., D. G. Evans, S. S. Kirkpatrick, and D. Y. Graham. 1991. Characterization of the Helicobacter pylori urease and purification of its subunits. Microb. Pathog. 10:15-26. [DOI] [PubMed] [Google Scholar]
- 12.Gergel, E. I., and M. B. Furie. 2001. Activation of endothelium by Borrelia burgdorferi in vitro enhances transmigration of specific subsets of T lymphocytes. Infect. Immun. 69:2190-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gergel, E. I., and M. B. Furie. 2004. Populations of human T lymphocytes that traverse the vascular endothelium stimulated by Borrelia burgdorferi are enriched with cells that secrete gamma interferon. Infect. Immun. 72:1530-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghiara, P., M. Rossi, M. Marchetti, A. Di Tommaso, C. Vindigni, F. Ciampolini, A. Covacci, J. L. Telford, M. T. De Magistris, M. Pizza, R. Rappuoli, and G. Del Giudice. 1997. Therapeutic intragastric vaccination against Helicobacter pylori in mice eradicates an otherwise chronic infection and confers protection against reinfection. Infect. Immun. 65:4996-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamlet, A., A. C. Thoreson, O. Nilsson, A. M. Svennerholm, and L. Olbe. 1999. Duodenal Helicobacter pylori infection differs in cagA genotype between asymptomatic subjects and patients with duodenal ulcers. Gastroenterology 116:259-268. [DOI] [PubMed] [Google Scholar]
- 16.Harris, P. R., P. B. Ernst, S. Kawabata, H. Kiyono, M. F. Graham, and P. D. Smith. 1998. Recombinant Helicobacter pylori urease activates primary mucosal macrophages. J. Infect. Dis. 178:1516-1520. [DOI] [PubMed] [Google Scholar]
- 17.Hatz, R. A., G. Meimarakis, E. Bayerdorffer, M. Stolte, T. Kirchner, and G. Enders. 1996. Characterization of lymphocytic infiltrates in Helicobacter pylori-associated gastritis. Scand. J. Gastroenterol. 31:222-228. [DOI] [PubMed] [Google Scholar]
- 18.Hatz, R. A., G. Rieder, M. Stolte, E. Bayerdorffer, G. Meimarakis, F. W. Schildberg, and G. Enders. 1997. Pattern of adhesion molecule expression on vascular endothelium in Helicobacter pylori-associated antral gastritis. Gastroenterology 112:1908-1919. [DOI] [PubMed] [Google Scholar]
- 19.Higuchi, K., T. Arakawa, T. Uchida, K. Nakagawa, S. Nakamura, T. Matsumoto, T. Fukuda, K. Kobayashi, and T. Kuroki. 1997. In situ expression of cell adhesion molecules in chronic gastritis with Helicobacter pylori infection. J. Clin. Gastroenterol. 25(Suppl. 1):S215-S221. [DOI] [PubMed] [Google Scholar]
- 20.Innocenti, M., A. M. Svennerholm, and M. Quiding-Jarbrink. 2001. Helicobacter pylori lipopolysaccharides preferentially induce CXC chemokine production in human monocytes. Infect. Immun. 69:3800-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Innocenti, M., A. C. Thoreson, R. L. Ferrero, E. Stromberg, I. Bolin, L. Eriksson, A. M. Svennerholm, and M. Quiding-Jarbrink. 2002. Helicobacter pylori-induced activation of human endothelial cells. Infect. Immun. 70:4581-4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jhala, N. C., G. P. Siegal, K. Klemm, B. F. Atkinson, and D. N. Jhala. 2003. Infiltration of Helicobacter pylori in the gastric mucosa. Am. J. Clin. Pathol. 119:101-107. [DOI] [PubMed] [Google Scholar]
- 23.Kim, C. H., L. Rott, E. J. Kunkel, M. C. Genovese, D. P. Andrew, L. Wu, and E. C. Butcher. 2001. Rules of chemokine receptor association with T-cell polarization in vivo. J. Clin. Investig. 108:1331-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunkel, E. J., J. J. Campbell, G. Haraldsen, J. Pan, J. Boisvert, A. I. Roberts, E. C. Ebert, M. A. Vierra, S. B. Goodman, M. C. Genovese, A. J. Wardlaw, H. B. Greenberg, C. M. Parker, E. C. Butcher, D. P. Andrew, and W. W. Agace. 2000. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192:761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwok, T., S. Backert, H. Schwarz, J. Berger, and T. F. Meyer. 2002. Specific entry of Helicobacter pylori into cultured gastric epithelial cells via a zipper-like mechanism. Infect. Immun. 70:2108-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindholm, C., M. Quiding-Jarbrink, H. Lonroth, A. Hamlet, and A. M. Svennerholm. 1998. Local cytokine response in Helicobacter pylori-infected subjects. Infect. Immun. 66:5964-5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuyama, N., T. Kirikae, F. Kirikae, M. Hashimoto, K. Amanot, S. Hayashi, Y. Hirai, T. Kubota, and M. Nakano. 2001. Non-standard biological activities of lipopolysaccharide from Helicobacter pylori. J. Med. Microbiol. 50:865-869. [DOI] [PubMed] [Google Scholar]
- 28.Michetti, M., C. P. Kelly, J. P. Kraehenbuhl, H. Bouzourene, and P. Michetti. 2000. Gastric mucosal α4β7-integrin-positive CD4 T lymphocytes and immune protection against helicobacter infection in mice. Gastroenterology 119:109-118. [DOI] [PubMed] [Google Scholar]
- 29.Mohan, K., Z. Ding, J. Hanly, and T. B. Issekutz. 2002. IFN-gamma-inducible T-cell alpha chemoattractant is a potent stimulator of normal human blood T lymphocyte transendothelial migration: differential regulation by IFN-gamma and TNF-alpha. J. Immunol. 168:6420-6428. [DOI] [PubMed] [Google Scholar]
- 30.Montecucco, C., and R. Rappuoli. 2001. Living dangerously: how Helicobacter pylori survives in the human stomach. Nat. Rev. Mol. Cell. Biol. 2:457-466. [DOI] [PubMed] [Google Scholar]
- 31.Montemurro, P., H. Nishioka, W. G. Dundon, M. de Bernard, G. Del Giudice, R. Rappuoli, and C. Montecucco. 2002. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a potent stimulant of mast cells. Eur. J. Immunol. 32:671-676. [DOI] [PubMed] [Google Scholar]
- 32.Oppenheimer-Marks, N., L. S. Davis, D. T. Bogue, J. Ramberg, and P. E. Lipsky. 1991. Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J. Immunol. 147:2913-2921. [PubMed] [Google Scholar]
- 33.O'Toole, P. W., S. M. Logan, M. Kostrzynska, T. Wadstrom, and T. J. Trust. 1991. Isolation and biochemical and molecular analyses of a species-specific protein antigen from the gastric pathogen Helicobacter pylori. J. Bacteriol. 173:505-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papadogiannakis, N., R. Willen, B. Carlen, S. Sjostedt, T. Wadstrom, and A. Gad. 2000. Modes of adherence of Helicobacter pylori to gastric surface epithelium in gastroduodenal disease: a possible sequence of events leading to internalisation. Apmis 108:439-447. [DOI] [PubMed] [Google Scholar]
- 35.Parsonnet, J., G. D. Friedman, D. P. Vandersteen, Y. Chang, J. H. Vogelman, N. Orentreich, and R. K. Sibley. 1991. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325:1127-1131. [DOI] [PubMed] [Google Scholar]
- 36.Pietschmann, P., J. J. Cush, P. E. Lipsky, and N. Oppenheimer-Marks. 1992. Identification of subsets of human T cells capable of enhanced transendothelial migration. J. Immunol. 149:1170-1178. [PubMed] [Google Scholar]
- 37.Quiding-Jarbrink, M., I. Ahlstedt, C. Lindholm, E. L. Johansson, and H. Lonroth. 2001. Homing commitment of lymphocytes activated in the human gastric and intestinal mucosa. Gut 49:519-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quiding-Jarbrink, M., B. S. Lundin, H. Lonroth, and A. M. Svennerholm. 2001. CD4+ and CD8+ T cell responses in Helicobacter pylori-infected individuals. Clin. Exp. Immunol. 123:81-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren, Z., G. Pang, R. Lee, R. Batey, M. Dunkley, T. Borody, and R. Clancy. 2000. Circulating T-cell response to Helicobacter pylori infection in chronic gastritis. Helicobacter 5:135-141. [DOI] [PubMed] [Google Scholar]
- 40.Roth, S. J., M. W. Carr, and T. A. Springer. 1995. C-C chemokines, but not the C-X-C chemokines interleukin-8 and interferon-gamma inducible protein-10, stimulate transendothelial chemotaxis of T lymphocytes. Eur. J. Immunol. 25:3482-3488. [DOI] [PubMed] [Google Scholar]
- 41.Sallusto, F. 1999. The role of chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Hematologica 84(Suppl. EHA 4):28-31. [PubMed] [Google Scholar]
- 42.Selbach, M., S. Moese, T. F. Meyer, and S. Backert. 2002. Functional analysis of the Helicobacter pylori cag pathogenicity island reveals both VirD4-CagA-dependent and VirD4-CagA-independent mechanisms. Infect. Immun. 70:665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Springer, T. A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76:301-314. [DOI] [PubMed] [Google Scholar]
- 44.Stromberg, E., A. Lundgren, A. Edebo, S. Lundin, A. M. Svennerholm, and C. Lindholm. 2003. Increased frequency of activated T cells in the Helicobacter pylori-infected antrum and duodenum. FEMS Immunol. Med. Microbiol. 36:159-168. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki, K., Y. Kokai, N. Sawada, R. Takakuwa, K. Kuwahara, E. Isogai, H. Isogai, and M. Mori. 2002. SS1 Helicobacter pylori disrupts the paracellular barrier of the gastric mucosa and leads to neutrophilic gastritis in mice. Virchows Arch. 440:318-324. [DOI] [PubMed] [Google Scholar]
- 46.Terres, A. M., J. M. Pajares, A. M. Hopkins, A. Murphy, A. Moran, A. W. Baird, and D. Kelleher. 1998. Helicobacter pylori disrupts epithelial barrier function in a process inhibited by protein kinase C activators. Infect. Immun. 66:2943-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]