Figure 3.
In Vitro Immunosuppressive and Anti-Inflammatory Properties of BM-MSCs from HDs and AML Patients
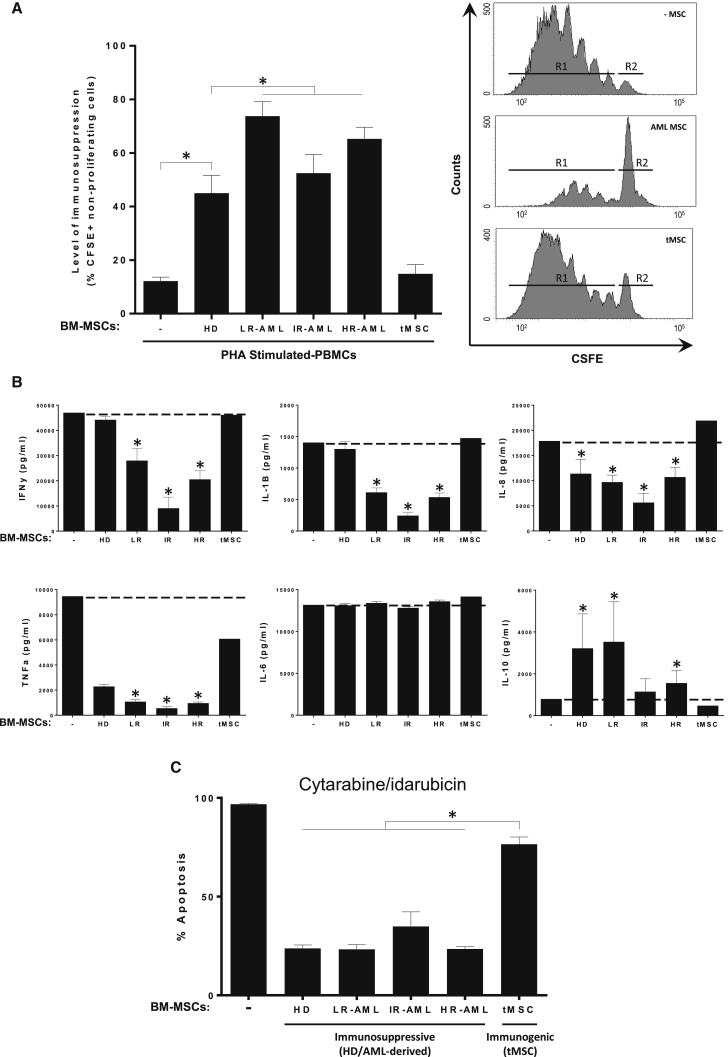
(A) Left: level of immunosuppression measured as percentage of CFSE+ non-proliferating cells. CSFE-labeled PBMCs were stimulated with PHA and then co-cultured with BM-MSCs from LR-, IR-, and HR-AML subjects for 5 days. BM-MSCs from HD and transformed BM-MSCs (tMSCs) were used as positive and negative controls, respectively. Right: the number of cycling (CSFEmild/low) cells was determined by flow cytometry. Representative flow-cytometry histograms of CSFE-labeled PBMCs: R1, proliferating cells; R2 non-proliferating cells. n = 35 patients (11 LR-, 10 IR-, and 14 HR-AML) and n = 10 healthy donor controls. ∗p < 0.05. Error bars indicate the SEM values of the biological replicates.
(B) Concentration of the indicated cytokines in cell-culture supernatants determined by Luminex Multiplex assays. PBMCs from HD were co-cultured with BM-MSCs from 10 HD, 2 tMSCs, and 35 BM-MSCs from LR-, IR-, and HR-AML subjects. Error bars indicate the SEM values of the biological replicates. ∗p < 0.05.
(C) Similar protective effect of immunosuppressive BM-MSCs from HD and AML (LR-, IR- and HR-) individuals, but not tMSCs, to Ara-C/idarubicin cytotoxic treatment of HL60 AML cells. Error bars indicate the SEM values of the biological replicates. ∗p < 0.05.