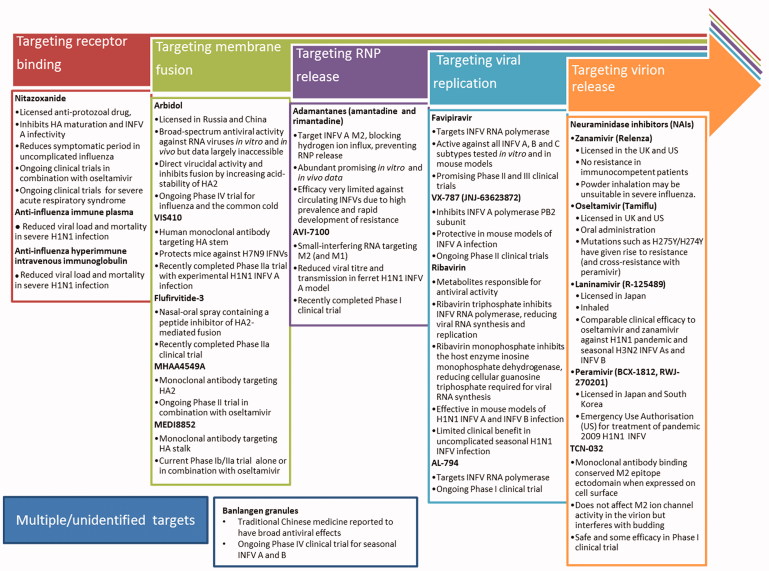
Figure 1.
Licensed and prospective antiviral therapeutics in clinical trials for influenza virus infection. The continual rise of resistance to current therapeutics necessitates the development of further anti-influenza drugs, targeted at multiple stages of the infection and replication cycle. Therapeutics described in Figure 1 are currently or have recently been under investigation in clinical trials registered with Clinicaltrials.gov. Therapeutics targeting receptor binding include nitazoxanide (Rossignol et al., 2009; reviewed in Rossignol, 2014), anti-influenza immune plasma (Luke et al., 2006; NIAID, 2010; Zhou et al., 2007; reviewed in Hui & Lee 2013) and anti-influenza hyperimmune intravenous immunoglobulin (Hung et al., 2013; NIAID, 2014). Therapeutics targeting membrane fusion include arbidol (Gagarinova et al., 1993; reviewed in Blaising et al., 2014), VIS410 (Tharakaraman et al., 2015; Visterra Inc, 2015;), Flufirvitide-3 (Autoimmune Technologies LLC [date unknown]; Autoimmune Technologies LLC, 2015), MHAA4549A (Genentech Inc., 2014; Lim et al., 2016; Nakamura et al., 2013), and MEDI8852 (Kallewaard et al., 2016; MedImmune LLC, 2015). Therapeutics targeting ribonucleoprotein release include the adamantanes, amantadine, and rimantadine (Davies et al., 1964; Wingfield et al., 1969; reviewed in Alves Galvão et al., 2014), and AVI-7100 (NIAID, 2012; reviewed in Dunning et al., 2014). Therapeutics targeting viral replication include favipiravir (Furuta et al., 2002; reviewed in Furuta et al., 2013), VX-787 (Clark et al., 2014; reviewed in Stevaert & Naesens, 2016), ribavirin (Durr et al., 1975; Eriksson et al. 1977; Smith et al., 1980; Smee et al., 2006) and AL-794 (Alios Biopharma Inc., 2015; reviewed in Blair & Cox, 2016). Therapeutics targeting virion release include neuraminidase inhibitors (Babu et al., 2000; Kim et al., 1997; von Itzstein et al., 1993; Yamashita et al., 2009; reviewed in Kamali & Holodniy 2013) and TCN-032 (Ramos et al., 2015). Therapeutics with unidentified mechanisms include banlangen granules (Hutchison Whampoa Guangzhou Baiyunshan Chinese Medicine Company Limited, 2012).