Abstract
Plasmodium falciparum MSP6 is a merozoite surface antigen that shows organization and sequence homologies similar to those of MSP3. Within its C-terminus conserved region, it presents some epitopes that are cross-reactive with MSP3 and others that are not, both being targets of naturally occurring antibodies that block the P. falciparum erythrocytic cycle in cooperation with monocytes.
Plasmodium falciparum MSP6 has been described recently as a merozoite surface molecule, structurally related in its overall sequence organization to the previously described MSP3 (10, 17). The C-terminal part of the protein shows homology with MSP3 (ca. 50% identity and 85% similarity of amino acid residues) and an identity for an 11-amino-acid (aa) stretch (ILGWEFGGG[A/V]P) previously identified as a target of antibodies with strong antiparasite activity (9, 13). Moreover, the C-terminal part of MSP6 is highly conserved in MSP6, as in the case of MSP3, whereas the N-terminal part displays polymorphisms, is proteolytically cleaved, and is less antigenic than the C-terminal part (10, 18).
MSP3 has been identified as a target of protective antibodies by assays involving antibody-dependent cellular inhibition (ADCI), a mechanism found to best reflect the protection that can be passively transferred by antibodies in P. falciparum-infected patients (9). MSP3 has been pursued for human vaccine trials based on the following series of findings suggesting that anti-MSP3 antibodies contribute to protection against malaria. (i) Immunoepidemiological studies showed a significant correlation of immunoglobulin G3 (IgG3) antibodies with protection acquired by natural exposure to the parasite (13, 14). (ii) Either naturally occurring or artificially raised antibodies have a strong monocyte-dependent ADCI effect (9, 13). (iii) Immunity can be actively elicited in primates against a P. falciparum challenge and correlates with prechallenge anti-MSP3 antibody titers (7). (iv) Immunity can be passively transferred by anti-MSP3 antibodies in P. falciparum-infected SCID mice (1, 13) and Plasmodium reichnowi-infected chimpanzees (M. Dziegel et al., submitted for publication).
Given the homologies of MSP6 with MSP3, we therefore performed a detailed study of the antigenicity of MSP6 and assessed the antiparasite role of the naturally occurring anti-MSP6 antibodies.
Antigenicity in populations of areas of endemicity.
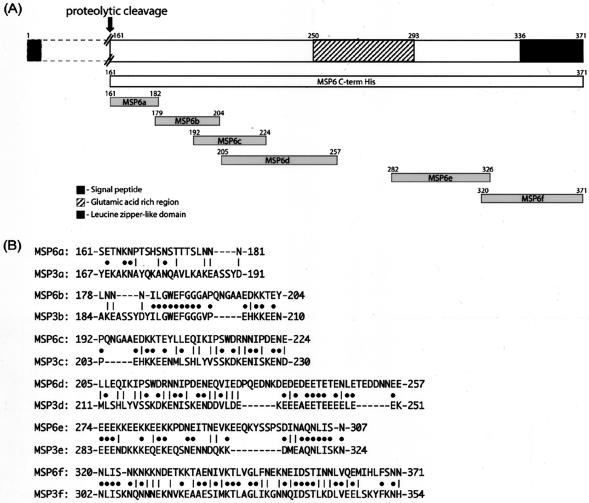
The C-terminal part of MSP6 (aa 161 to 371 in clone 3D7) was cloned and expressed as a recombinant histidine-tagged protein (MSP6-Cterm), as described earlier (16). Six overlapping peptides (MSP6a161-182, MSP6b179-204, MSP6c192-224, MSP6d205-257, MSP6e282-326, and MSP6f320-371) were designed in a similar manner to those from MSP3 (13), each representing a different region of the C-terminal part, as shown in Fig. 1. A small glutamic acid-rich region (aa 258 to 281; 54% glutamic acid) was excluded to avoid cross-reactivity exhibited by glutamate-rich epitopes present in several P. falciparum antigens (8). Enzyme-linked immunosorbent assays (ELISAs) were performed as described earlier (13) to determine the level of total IgG and the subclass distribution against each peptide in sera from 30 malaria-protected African adults from Ivory Coast. Passive transfer of IgG purified from these sera was earlier found to markedly reduce the level of parasitemia in malaria patients (12).
FIG. 1.
(A) Schematic presentation of P. falciparum MSP6 protein and the design of MSP6-Cterm recombinant protein and peptides (MSP6a, MSP6b, MSP6c, MSP6d, MSP6e, and MSP6f). The representation of the N-terminal part of the molecule is compressed here (indicated by dotted lines). The numbers show amino acid positions for each region based on the sequence derived from strain 3D7. (B) Homologous alignment of different MSP6 peptide regions with their corresponding regions from MSP3. Solid circles represent identity, while vertical lines show similarity of the amino acid residues shared between the two related molecules (using the Wilbur-Lipman algorithm for pairwise alignment, PAM250).
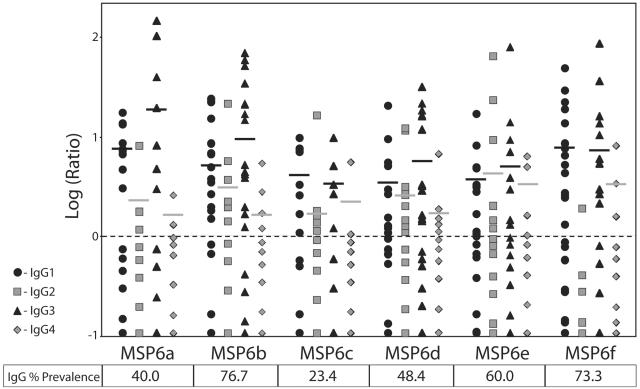
Figure 2 summarizes antibody subclass reactivity recorded against the six peptides covering the various regions of MSP6-Cterm. Though the prevalence of IgG against different regions varied, the pattern of antibody subclass reactivity to each peptide was rather homogeneous, with an overall dominance of cytophilic antibodies IgG1 and IgG3. Substantial levels of IgG2 antibodies were also detected against some of the peptides, e.g., MSP6e. This antibody subclass pattern differs from that observed against several blood-stage antigens. For instance, the corresponding regions of MSP3 showed a predominance of IgG3 against MSP3b, MSP3c, and MSP3d peptides, whereas IgG1 predominated against MSP3f (13). Differential patterns of antibody subclass have also been found against distinct regions within a single protein—e.g., a predominance of IgG3 against MSP1 Block 2 in contrast to IgG1 against MSP119 (3)—and between different proteins—e.g., predominance of IgG3 against MSP2 (11, 15) as compared to IgG1 against RAP-1 (4) and AMA-1 (S. Singh et al., unpublished results). Within the family of another merozoite surface antigen, IgG3 is predominant against MSP4 as compared to the predominance of IgG1 against MSP5 (18, 19). The factors responsible for a distinct human subclass response to different antigens are not fully understood: however, the nature of the antigen itself (6) and the cytokine milieu experienced by the responding B cells (5) may both influence the outcome.
FIG. 2.
Prevalence and titer of antibodies against different regions of MSP6 in hyperimmune sera (n = 30) from Ivory Coast. Antibody reactivity was considered to be positive if the ratio of the mean OD of the test sera to the mean OD of the control sera + 3 × the standard deviation of the control sera was ≥1. The figure represents antibody titers, expressed as a ratio for each serum, and the dotted line represents the baseline of a ratio equal to 1. The horizontal bars represent the arithmetic mean level of each isotype for positive sera. The table shows the prevalence of sera positive for total IgG to different regions of MSP6.
Antimalarial activity of anti-MSP6 antibodies.
To assess the functional activity of human antibodies towards different regions of MSP6, we affinity purified antibodies against each of the six peptides, using independent serum pools (each made up of five to seven individual serum samples) selected as described earlier (13) on the basis of high content of cytophilic antibodies (IgG1 plus IgG3) and minimal reactivity towards the adjacent regions. The affinity-purified antibodies proved to be specific against the respective peptides, as no cross-reactivity was observed to other regions of the molecule (Table 1, shown by optical density at 450 nm [OD450]). Thus, each of the MSP6 peptides was found to define at least one B-cell epitope that does not share antigenic determinants with other regions of the molecule. Indirect immunofluorescence assays (IFA) on acetone-fixed thin smears of P. falciparum asexual blood-stage parasites indicated that each antipeptide antibody was reactive with the native parasite protein (data not shown).
TABLE 1.
Specificity of affinity-purified human anti-MSP6 antibodies determined by ELISA
| Region | Mean OD450 for antibody toa:
|
|||||
|---|---|---|---|---|---|---|
| MSP6a | MSP6b | MSP6c | MSP6d | MSP6e | MSP6f | |
| Specificity | ||||||
| MSP6a | 0.118 | 0.009 | 0.008 | 0.008 | 0.009 | 0.008 |
| MSP6b | 0.007 | 0.111 | 0.007 | 0.008 | 0.007 | 0.009 |
| MSP6c | 0.009 | 0.008 | 0.084 | 0.018 | 0.006 | 0.007 |
| MSP6d | 0.010 | 0.007 | 0.008 | 0.116 | 0.007 | 0.009 |
| MSP6e | 0.009 | 0.009 | 0.009 | 0.008 | 0.085 | 0.008 |
| MSP6f | 0.007 | 0.008 | 0.007 | 0.008 | 0.009 | 0.092 |
| Cross-reactivity | ||||||
| MSP3a | 0.007 | 0.007 | 0.008 | 0.010 | 0.007 | 0.012 |
| MSP3b | 0.010 | 0.105 | 0.008 | 0.008 | 0.009 | 0.007 |
| MSP3c | 0.008 | 0.009 | 0.010 | 0.008 | 0.008 | 0.009 |
| MSP3d | 0.010 | 0.009 | 0.007 | 0.049 | 0.008 | 0.008 |
| MSP3e | 0.008 | 0.008 | 0.009 | 0.009 | 0.045 | 0.010 |
| MSP3f | 0.009 | 0.009 | 0.011 | 0.009 | 0.008 | 0.085 |
Antibodies affinity purified against different regions of MSP6 were tested for specificity and cross-reactivity to related regions in MSP3. Mean OD450s from duplicate wells are shown. All of the peptides were used under identical coating conditions. Boldface values represent positive reactivity.
The antiparasite activity was thereafter assessed in vitro with monocyte-dependent ADCI assays. To this end, each peptide-specific antibody was adjusted to an equal effective concentration by testing reactivity to the parasite protein (1/200 IFA endpoint titer), as previously described (13) for testing in ADCI assays. The assays were performed in duplicates over a 96-h period, using the 3D7 strain of parasites and monocytes from healthy donors, as described earlier (2). The affinity-purified antibodies, dialyzed against RPMI medium, were added at a ratio of 10% (vol/vol) of the complete culture medium; thus, each of them was used at a final IFA titer of 1/20 in the ADCI assay. Parasitemia, determined at the end of 96 h by microscopic examination of ≥10,000 erythrocytes on Giemsa-stained thin smears, was used to calculate the specific growth inhibitory index (SGI) as follows: % SGI = 1 − [(% parasitemia with monocytes and test IgG/% parasitemia with test IgG)/(% parasitemia with monocytes and normal IgG/% parasitemia with normal IgG)] × 100. Results from the ADCI assays (Fig. 3) show that antibodies affinity purified against each of the six peptides were able to exert a strong monocyte-dependent inhibition of the parasite growth. This result differs markedly from those obtained with MSP3, where only three of the six peptide-specific antibodies were effective (13). No significant direct effect upon parasite growth (in the absence of monocytes) was observed at the antibody concentrations employed (data not shown).
FIG. 3.
Effect of human affinity-purified anti-MSP6 antibodies on parasite growth in ADCI assays. The histograms represent mean values of percent SGI (as explained in the text) from three independent experiments ± standard error; values >30% were considered significant. PIAG, positive control IgG from the pool of Ivory Coast adult sera used for passive transfer in humans (10). The level of parasite inhibition obtained with affinity-purified antibodies was adjusted in proportion to the effect observed by PIAG, which was considered to be 100%. NIgG, negative control IgG from pool of French donors not exposed to malaria. Anti-RESA antibodies were affinity purified from a pool of hyperimmune sera (Ivory Coast) against a synthetic peptide (sequence H-[EENVEHDA]2-[EENV]2-OH).
MSP6 and MSP3 share cross-reactive epitopes.
Cross-reactivity was examined by using anti-MSP6 affinity-purified antibodies against homologous peptides from MSP3. As shown in Table 1, four regions were found to be cross-reactive. Anti-MSP6b and -f antibodies were fully cross-reactive, whereas anti-MSP6d and -e antibodies displayed partial cross-reactivity. In contrast, anti-MSP6a and anti-MSP6c did not show cross-reactivity to the corresponding MSP3 regions. These results are in overall agreement with the sequence homologies (Fig. 1B) and suggest that the antiparasite effect mediated by some of the anti-MSP6 antibodies could also be due to the binding to cross-reactive regions in MSP3. However, parasite inhibition mediated by non-cross-reactive MSP6 antibodies, such as anti-MSP6a and anti-MSP6c, demonstrate that MSP6 is also a target of ADCI on its own.
This study demonstrates that, together with considerable sequence similarities which result in sharing of antigenic properties, there exist striking differences between various regions of MSP6 as compared to MSP3. The observed patterns of antibody subclass reactivity and cross-reactivity against various regions of MSP3 and MSP6 indicate that the amino acid sequence divergences also result in true antigenic differences.
Both molecules have very valuable features, and effective antibodies can be induced by either one of them. In addition, antibodies directed to the cross-reactive epitopes can react with both molecules on the merozoite surface and this increases the number of antigenic targets for the effective antibodies. In contrast, the range of immune responses induced by MSP6 is wider than that for MSP3, which could provide a strategic advantage of using MSP6 as an immunogen rather than MSP3 as the former could induce antibodies reactive to both (i) molecules and (ii) non-cross-reactive epitopes, which also have valuable features in defense mechanisms.
The rationale that was applied to conduct MSP3 clinical trials obviously also applies to MSP6 in view of these features. However, only results from clinical trials with each antigen will substantiate whether MSP6, in practical terms, holds a strategic advantage over MSP3 in inducing in humans a larger variety of antibodies that mediate parasite killing.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Badell, E., C. Oeuvray, A. Moreno, S. Soe, N. van Rooijen, A. Bouzidi, and P. Druilhe. 2000. Human malaria in immunocompromised mice: an in vivo model to study defense mechanisms against Plasmodium falciparum. J. Exp. Med. 192:1653-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouharoun-Tayoun, H., P. Attanath, A. Sabchareon, T. Chongsuphajaisiddhi, and P. Druilhe. 1990. Antibodies which protect man against P. falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro but act in co-operation with monocytes. J. Exp. Med. 172:1633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavanagh, D. R., C. Dobaño, I. M. Elhassan, K. Marsh, A. Elhassan, L. Hviid, E. A. T. G. Khalil, T. G. Theander, D. E. Arnot, and J. S. McBride. 2001. Differential patterns of human immunoglobulin G subclass responses to distinct regions of a single protein, the merozoite surface protein 1 of Plasmodium falciparum. Infect. Immun. 69:1207-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonjungo, P. N., I. M. Elhassan, D. R. Cavanagh, T. G. Theander, L. Hviid, C. Roper, D. E. Arnot, and J. S. McBride. 1999. A longitudinal study of human antibody responses to Plasmodium falciparum rhoptry-associated protein 1 in a region of seasonal and unstable malaria transmission. Infect. Immun. 67:2975-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garraud, O., and T. B. Nutman. 1996. The roles of cytokines on human B-cell differentiation into immunoglobulin secreting cells. Bull. Inst. Pasteur 94:285-309. [Google Scholar]
- 6.Garraud, O., R. Perraut, A. Diouf, W. S. Nambei, A. Tall, A. Spiegel, S. Longacre, D. C. Kaslow, H. Jouin, D. Mattei, G. M. Engler, T. B. Nutman, E. M. Riley, and O. Mercereau-Puijalon. 2002. Regulation of antigen-specific immunoglobulin G subclasses in response to conserved and polymorphic Plasmodium falciparum antigens in an in vitro model. Infect. Immun. 70:2820-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hisaeda, H., A. Saul, J. J. Reece, M. C. Kennedy, C. A. Long, L. H. Miller, and A. W. Stowers. 2002. Merozoite surface protein-3 and protection against malaria in Aotus nancymai monkeys. J. Infect. Dis. 185:657-664. [DOI] [PubMed] [Google Scholar]
- 8.Mattei, D., K. Berzins, M. Wahlgren, R. Udomsangpetch, P. Perlmann, H. W. Griesser, A. Scherf, B. Muller-Hill, S. Bonnefoy, M. Guillotte, G. Langsley, L. H. Pereira da Silva, and O. Mercereau-Puijalon. 1989. Cross-reactive antigenic determinants present on different Plasmodium falciparum blood-stage antigens. Parasite Immunol. 11:15-29. [DOI] [PubMed] [Google Scholar]
- 9.Oeuvray, C., H. Bouharoun-Tayoun, H. Gras-Masse, E. Bottius, T. Kaidoh, M. Aikawa, M. C. Filgueira, A. Tartar, and P. Druilhe. 1994. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by co-operation with blood monocytes. Blood 84:1594-1602. [PubMed] [Google Scholar]
- 10.Pearce, J. A., T. Triglia, A. N. Hodder, D. C. Jackson, A. F. Cowman, and R. F. Anders. 2004. Plasmodium falciparum merozoite surface protein 6 is a dimorphic antigen. Infect. Immun. 72:2321-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rzepczyk, C. M., K. Hale, N. Woodroffe, A. Bobogare, P. Csurhes, A. Ishii, and A. Ferrante. 1997. Humoral immune responses of Solomon Islanders to the merozoite surface antigen 2 of Plasmodium falciparum show pronounced skewing towards antibodies of the immunoglobulin G3 subclass. Infect. Immun. 65:1098-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabchareon, A., T. Burnouf, D. Ouattara, P. Attanath, H. Bouharoun-Tayoun, P. Chantavanich, C. Foucault, T. Chongsuphajaisiddhi, and P. Druilhe. 1991. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hyg. 45:297-308. [DOI] [PubMed] [Google Scholar]
- 13.Singh, S., S. Soe, J. P. Mejia, C. Roussilhon, M. Theisen, G. Corradin, and P. Druilhe. 2004. Identification of a conserved region of Plasmodium falciparum MSP3 targeted by biologically active antibodies to improve vaccine design. J. Infect. Dis. 190:1010-1018. [DOI] [PubMed] [Google Scholar]
- 14.Soe, S., M. Theisen, C. Roussilhon, K.-S.-Aye, and P. Druilhe. 2004. Association between protection against clinical malaria and antibodies to merozoite surface antigens in an area of hyperendemicity in Myanmar: complementarity between responses to merozoite surface protein 3 and the 220-kilodalton glutamate-rich protein. Infect. Immun. 72:247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor, R. R., D. B. Smith, V. J. Robinson, J. S. McBride, and E. M. Riley. 1995. Human antibody response to Plasmodium falciparum merozoite surface protein 2 is serogroup specific and predominantly of the immunoglobulin G3 subclass. Infect. Immun. 63:4382-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theisen, M., J. Vuust, A. Gottschau, S. Jespen, and B. Høgh. 1995. Antigenicity and immunogenicity of recombinant glutamate-rich protein of Plasmodium falciparum expressed in Escherichia coli. Clin. Diagn. Lab. Immunol. 2:30-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trucco, C., D. Fernandez-Reyes, S. Howell, W. H. Stafford, T. J. Scott-Finnigan, M. Grainger, S. A. Ogun, W. R. Taylor, and A. A. Holder. 2001. The merozoite surface protein 6 gene codes for a 36 kDa protein associated with the Plasmodium falciparum merozoite surface protein-1 complex. Mol. Biochem. Parasitol. 112:91-101. [DOI] [PubMed] [Google Scholar]
- 18.Wang, L., L. Crouch, T. L. Richie, D. H. Nhan, and R. L. Coppel. 2003. Naturally acquired antibody responses to the components of the Plasmodium falciparum merozoite surface protein 1 complex. Parasite Immunol. 25:403-412. [DOI] [PubMed] [Google Scholar]
- 19.Weisman, S., L. Wang, H. Billman-Jacobe, D. H. Nhan, T. L. Richie, and R. L. Coppel. 2001. Antibody responses to infections with strains of Plasmodium falciparum expressing diverse forms of merozoite surface protein 2. Infect. Immun. 69:959-967. [DOI] [PMC free article] [PubMed] [Google Scholar]