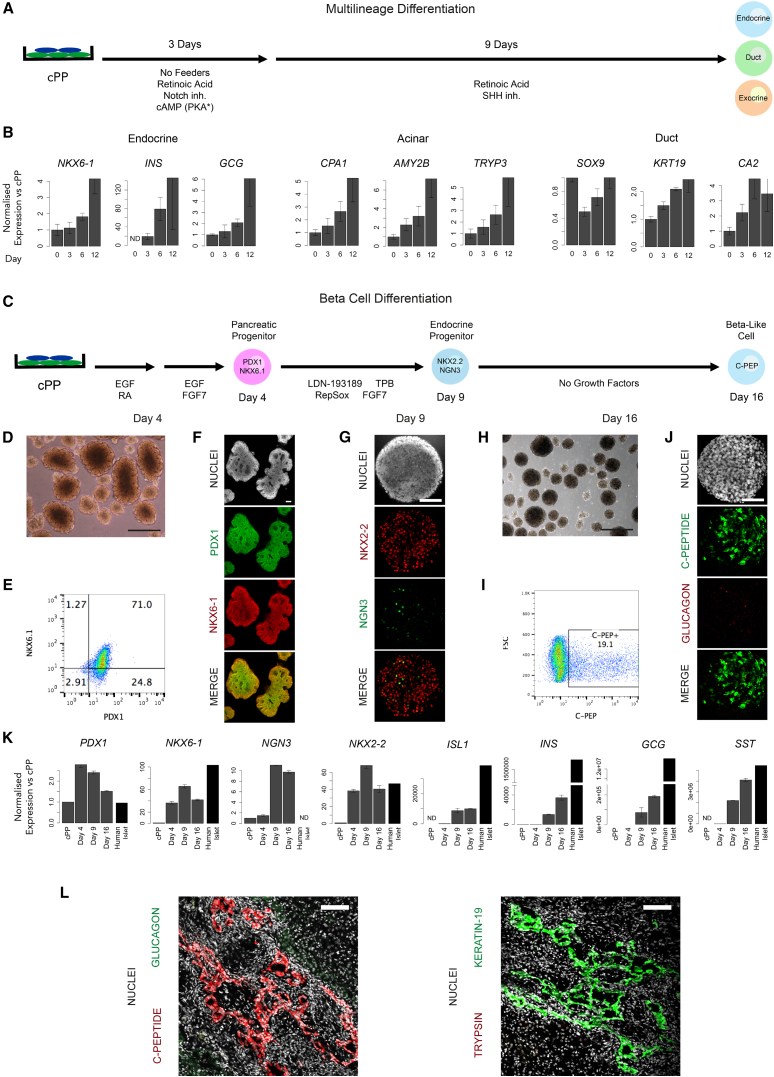
Figure 5.
Testing cPP Potency In Vitro and In Vivo
(A) Feeder-depleted passage 15 H9 cPP cells were replated on Matrigel and exposed to the indicated factors that promote multilineage differentiation toward the endocrine, duct, and acinar lineages.
(B) Endocrine, exocrine, and ductal gene expression analysis in (A) after 3, 6, and 12 days. Values are shown relative to levels in undifferentiated cPP cells (day 0). Error bars represent the SE of three technical replicates.
(C) Directed differentiation of passage 10 AK6-13 cPP cells to insulin+ β-like cells using a modified version of Russ et al. (2015).
(D) Phase-contrast image of differentiating spheres undergoing branching morphogenesis after 4 days. Scale bar, 100 μm.
(E) Intracellular flow cytometric analysis of day 4 cells shows approximately 70% reactivate NKX6-1 and maintain PDX1.
(F) PDX1 and NKX6-1 immunostaining on day 4. Scale bar, 100 μm.
(G) On day 9, the majority of cells are NKX2-2+ with a proportion of these transiently NGN3+. Scale bar, 100 μm.
(H) Phase contrast image of day 16 spheres. Scale bar, 100 μm.
(I) Approximately 20% of cells are C-peptide+ on day 16.
(J) Day 16 C-peptide+ cells do not coexpress glucagon. Scale bar, 100 μm.
(K) Gene expression measured by qRT-PCR of cPP cells on days 4, 9, and 16 harvested from the differentiation protocol in (C). Levels are shown relative to those in undifferentiated cPP cells and human islets for comparison. Error bars represent the SE of three technical replicates.
(L) Immunostaining of transplanted cPP cells for markers of endocrine (C-peptide and glucagon), duct (keratin-19), and acinar (trypsin) lineages. Scale bar, 100 μm.