Abstract
BACKGROUND
Chemotherapy-induced peripheral neuropathy (CIPN) is a significant problem for cancer patients, and there are limited treatment options for this often debilitating condition. Neuromodulatory interventions could be a novel modality for patients trying to manage CIPN symptoms; however, they are not yet the standard of care. This study examined whether electroencephalogram (EEG) neurofeedback (NFB) could alleviate CIPN symptoms in survivors.
METHODS
This was a randomized controlled trial with survivors assigned to an NFB group or a wait-list control (WLC) group. The NFB group underwent 20 sessions of NFB, in which visual and auditory rewards were given for voluntary changes in EEGs. The Brief Pain Inventory (BPI) worst-pain item was the primary outcome. The BPI, the Pain Quality Assessment Scale, and EEGs were collected before NFB and again after treatment. Outcomes were assessed with general linear modeling.
RESULTS
Cancer survivors with CIPN (average duration of symptoms, 25.3 mo), who were mostly female and had a mean age of 62.5 years, were recruited between April 2011 and September 2014. One hundred percent of the participants starting the NFB program completed it (30 in the NFB group and 32 in the WLC group). The NFB group demonstrated greater improvement than the controls on the BPI worst-pain item (mean change score, −2.43 [95% confidence interval, −3.58 to −1.28] vs 0.09 [95% confidence interval, −0.72 to −0.90]; P 5 .001; effect size, 0.83).
CONCLUSIONS
NFB appears to be effective at reducing CIPN symptoms. There was evidence of neurological changes in the cortical location and in the bandwidth targeted by the intervention, and changes in EEG activity were predictive of symptom reduction.
Keywords: chemotherapy-induced neuropathy, electroencephalogram (EEG), neurofeedback, neuromodulation, pain
INTRODUCTION
Chemotherapy-induced peripheral neuropathy (CIPN) is a common side effect of cytotoxic cancer therapy and has been described as one of the most debilitating because it negatively affects quality of life and function. The vast majority of cancer survivors who receive neurotoxic chemotherapeutic agents (taxanes, platinums, vinca alkaloids, and bortezomib) develop CIPN at some point during therapy.1,2 The incidence of CIPN 1 month after chemotherapy is estimated to be as high as 71% to 96%.3 Most survivors will have some resolution of symptoms over time4; however, even 6 months after treatment, 30% of those receiving platinum-based chemotherapies and as many as 80% of those receiving paclitaxel therapy continue to suffer from CIPN.4,5 To date, most treatments for CIPN are not effective, and the mechanisms by which these clinical impairments are alleviated remain allusive.6 In fact, 15 trials sponsored by the National Cancer Institute showed that only 1 pharmaceutical agent, duloxetine, provided positive results, whereas agents in the remaining 14 trials failed to treat or prevent neuropathic symptoms.6–8 With the increasing numbers of cancer survivors, there is an urgency for finding effective treatments for chronic CIPN.
Although it is common knowledge that there are certain brain regions and pathways activated in a variety of pain conditions (sometimes called the pain matrix), we do not have an effective understanding of when and how to intercept those pathways to alleviate pain. Techniques for pain modification include cognitive behavioral therapy, hypnosis, distraction, and coping skills training.9–11 There is, however, growing interest in brain/computer interface techniques; the idea is that modification of the source of the pain perception (the brain) will relieve symptoms.12–14 One of these techniques, electroencephalogram (EEG) neurofeedback (NFB), has successfully treated a variety of health issues, including pain conditions.15,16 NFB takes advantage of the learning process by providing feedback to the participant about the workings of his or her own brain in real time. Sensors are placed on the participant’s scalp at appropriate locations. Participants choose a game that rewards them for changing their brainwave activity under the sensors. Feedback is both auditory and visual (an emotionally neutral picture appears on the screen and is accompanied by a simultaneous auditory beep when participants successfully modify their brain activity). By targeting changes in brain regions that are active during pain conditions such as CIPN, NFB can teach participants to interpret pain signals differently.
Because CIPN is a significant problem for cancer survivors with very limited effective treatments, we conducted a trial to compare a neuromodulatory intervention (NFB) with a usual-care control (wait-list control [WLC]) with the primary outcome being pain related to CIPN. We hypothesized that NFB participants would demonstrate both significant reductions in neuropathic symptoms in comparison with those in the WLC group and changes in neural activity associated with the specificity of NFB training protocols and that changes in neural activity would predict changes in CIPN symptoms.
MATERIALS AND METHODS
Study Design
The current randomized, wait list–controlled trial investigated the efficacy of NFB at treating CIPN, examined the neural correlates associated with NFB, and explored whether changes in brain electrical activity predicted changes in CIPN. The study was approved by the institutional review board of The University of Texas MD Anderson Cancer Center (MDACC) in Houston, Texas. Measurements were taken at the baseline and within 5 days of the final session of NFB.
Participants
Participants were recruited from the MDACC database of survivors as well as MDACC physician referrals. The diagnosis of CIPN was made by the attending oncologist on the basis of the symptom history and a physical examination. Written consent was obtained before participation. The inclusion criteria were as follows: participants were 18 years old or older, had at least a grade 3 neuropathy rating according to the National Cancer Institute’s grading criteria (version 4, 2010) and/or reported CIPN-related moderate to severe pain (score of 4 or higher) on a numeric rating scale (0–10, with 10 being the worst), and had symptoms of neuropathy for a minimum of 3 months after the completion of chemotherapy. Participants with any cancer type or stage were eligible. Participants with neuropathic symptoms from any cause other than chemotherapy (eg, diabetes) were excluded. Other exclusion criteria included central nervous system disease or a history of bipolar disorder, schizophrenia, or antipsychotic medication usage. Concurrent uses of medications to treat neuropathic pain were allowed, but an increase in medications or changes in the types of medications were not allowed during the course of NFB treatment.
Procedures
The NFB group received 20 sessions of NFB over a maximum of 10 weeks; this required minimum participation of twice weekly. To eliminate the likelihood of symptoms improving because of the passage of time, we obtained data from the WLC group and the NFB group at the same time points. The WLC group was offered NFB once the study was complete. To ensure that we were measuring pain related to CIPN, our study participants were specifically instructed to rate their pain with respect to neuropathy (and not back pain, for example). In addition, the oncologist had to agree that the participant’s symptoms were related to CIPN.
EEG Recording
An EEG was recorded for each patient with an amplifier (Mitstar, St. Petersburg, Russia). The data were collected with a 19-electrode EEG cap, cleared of all artifacts, and processed offline (Eureka! software; NovaTech EEG). Data were collected for 10 minutes in the eyes-opened condition and in the eyes-closed condition (sampling rate, 256 Hz); the band passed from 0.5 to 64 Hz. Impedances were reduced below 5 kΩ. Participant data were then processed to result in a quantitative electroencephalogram (qEEG), from which NFB training protocols were derived.
NFB
Participants were seated in an upright position in a comfortable chair and instructed to watch the computer monitor as if they were watching a television. Electrodes were placed on each participant’s scalp at appropriate locations as designated by the qEEG-based protocols. The NFB challenge was such that participants were required to keep the amplitude of a desired EEG waveform above a certain threshold while inhibiting the amplitude of other, less desired waveforms. Participants played a game lasting 45 minutes, which consisted of an emotionally neutral picture appearing on the screen that was accompanied by a simultaneous auditory beep when a participant matched the thresholds (EEG amplitudes, for example) programmed in the software and determined by the qEEG. When a participant did not match the thresholds programmed in the software, the game paused, and no auditory or visual feedback was given. Feedback occurred approximately 78% of the time that the participants increased the amplitude of their EEG reward band (ie, when they increased it 8–12 Hz [or α]) while decreasing the amplitude of the inhibit band or bands. For NFB, we used EEGer software (EEG Education and Research, Granada Hills, Calif).
Measures
Pain
For the primary aim, the Brief Pain Inventory (BPI) short form was used to assess the severity of pain and its impact on daily functioning.17 The BPI short form is a validated, self-administered questionnaire widely used to assess the severity of pain and the impact of pain on daily functioning among patients. The BPI was designed to measure 2 key aspects of pain: sensory and reactive. Both dimensions use a 0 to 10 numeric rating scale, with 10 being the worst. A composite of 4 pain items is represented by the pain severity score, whereas a composite of 7 daily activities is represented by the pain interference scale.18 We chose the worst-pain subscale of the BPI as our primary outcome because it has been used as an outcome measure in trials of treatments for CIPN, has a high degree of reliability and validity, and has been shown to be valid when it is used as a single item.18
Secondary endpoints were changes in other CIPN-related symptoms, such as average pain, pain severity, activity interference, and affective interference (BPI) and pain unpleasantness, tingling, numbness, intensity, tenderness, surface pain, and deep pain (measured with the Pain Quality Assessment Scale [PQAS]). The PQAS is a 20-item measure developed to quantify the quality and intensity of neuropathic pain. It was derived from the Neuropathic Pain Scale and includes descriptors common to people with neuropathic pain.19 Items are scored as standalone measures, and a global score is not recommended. Participants complete rating scales from 0 to 10 to describe their experience over the past week on average.
Clinically important change has been defined as a raw point decrease ranging from 1.74 to 2 with the BPI.1 Considering that other studies have set a precedent of a change as low as 0.98 as a minimally important difference,1,18 we chose a 2.0-point decrease in the symptom report to be our definition of clinically meaningful.
EEG neuroimaging
The qEEG analysis included absolute and normalized spectra and a comparison of individual data with a normative database (Institute of the Human Brain, St. Petersburg, Russia). The qEEG was reviewed in 6 frequency bands: δ (1.5–3.5), θ (4.0–7.5), α (8.0–12.0), low β (13.0–16.0), high β (13.0–32.0), and γ (35.0–45.0). The qEEG was the basis for the NFB protocol design, for which protocols were created by the comparison of statistics from the normative database with the statistics from participants on an individual basis. We used low-resolution electromagnetic tomography (LORETA) to evaluate neural correlates of the placebo response to NFB.20
LORETA has been validated in studies that have combined LORETA with other established localization methods such as functional structural magnetic resonance imaging, structural magnetic resonance imaging, positron emission tomography, diffusion spectral imaging, and localization findings from invasive, implanted depth electrodes.21–24
Statistical Analysis
Subjective report of neuropathy
With a sample size of 30 evaluable participants per group (assuming a 20% attrition rate), we had 80% power to detect differences that were at least 0.74 standard deviations between groups. Because this was a pilot study, we had no preliminary data to suggest what a realistic between-group difference (or corresponding effect size) would be. However, the power analysis adds support because it allows a good chance of detecting a medium to large effect size. To identify changes in symptom reports associated with NFB, we performed a general linear model regression (GLM) controlling for age, baseline scores, the current use of medication for neuropathy, and the length of time with neuropathic symptoms with NFB versus WLC as a between-subjects factor. The GLM demonstrates that the observed data can be predicted from the model with 1 or more predictors and a continuous response variable.25 Effect sizes were computed through a comparison of pre-and postchange scores between groups with Cohen’s d.26 The primary study endpoint was the change in worst pain (BPI) from the baseline to the end of the treatment period (20 NFB sessions). Secondary endpoints were changes in other CIPN-related symptoms measured with the BPI and PQAS, and they were also calculated with our GLM. In summary, our dependent variable was the change in worst pain as measured by the BPI; our independent variable was the group; and our covariates were age, baseline scores, current use of medication for neuropathy, and length of time with neuropathic symptoms. Patients were randomized by minimization and assigned to a group via a computer. Participants were treated per protocol, and analyses were performed with IBM SPSS Statistics for Windows (version 22.0).
EEG neuroimaging
Neuroimaging targeted 3 outcomes: patterns of activation in 9 predesignated areas of the cortex (frontal left, midline, right, central [left, midline, and right], and posterior [left, midline, and right]) to determine cortical changes based on NFB, regions correlated with the worst-pain change scores to identify potential mechanisms of training-related changes, and an evaluation of 3 regions known to be active in placebo analgesia to determine to what degree the effects of NFB could be attributed to a placebo.
EEG records were manually artifacted and analyzed with Eureka! software (NovaTech EEG). The mean amplitude, power, and absolute values were exported into SPSS for statistical analysis. When it was applicable, the data were transformed with Ln(x) or Ln(x + 1) to produce a more Gaussian distribution. We confirmed that our data were normalized by an examination of Q-Q box plots before the analysis. θ/β and α/β ratios were calculated. A multivariate analysis (analysis of variance; group × time and group × time × region) was conducted to assess baseline and pre-post differences between groups for each frequency band and measure (eyes closed and relative power).25 Our dependent variable was relative EEG power. To correct for multiple comparisons, Bonferroni corrections were applied.27 We report relative power indices because relative power shows smaller interindividual variability than absolute power does28; this potentially allows a more accurate comparison of the power indices between epochs and electrodes, and they are commonly used to design neuromodulatory approaches to treating pain conditions. To explore the association between brain activity and NFB associated with the modulation of pain, a Pearson correlational analysis was performed between the cortical amplitude, power, relative power, and α/β power ratios and the change scores for worst pain.
Because this study did not have a placebo arm, we explored changes in regions of interest (ROIs) known to be active with a placebo and to what degree effects of NFB could be attributed to a placebo. The current source density was averaged across all voxels in an ROI, and we controlled for baseline activation across all frequency bands; then, the current source density activity was compared between groups. Our predetermined ROIs included the insula, the anterior cingulate cortex, and the dorsolateral prefrontal cortex. These areas have been shown to be active with placebo analgesia and to contribute to the descending pain control system.
RESULTS
Seventy-five patients were approached for the study between April 2011 and September 2014, and 71 participants were randomized. Five dropped out from the NFB group before any NFB sessions and 4 dropped out from the WLC group before follow-up for a total participation of 30 patients in the NFB and 32 patients in the control group. Of the 30 participants in the NFB group who started treatment, 100% completed all 20 sessions and provided follow-up data. The average age of the participants was 62.5 years; most were female (87%) and white (77.5%); and most were breast cancer survivors (73%) with an average length of time since chemotherapy of 25.3 months. Six participants in the NFB group and 6 in the WLC group did not report a pain rating of at least 4 points as measured by the BPI at the baseline. Fifty-one percent were not taking a medication to treat neuropathic symptoms at the time of study entry, and the chemotherapy regimens mainly involved a taxane agent (72%; Table 1). There were no statistically significant differences between groups at the baseline in age, length of time of neuropathic symptoms, type of chemotherapy, stage of disease, ethnicity, employment or marital status, education level, disease type, or percentage taking medications for CIPN or in any of the outcome measures.
TABLE 1.
Demographics and Baseline Features
| Treatment (n = 35) | Control (n = 36) | Total (n = 71) | |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD), ya | 62 (9.6) | 63 (11) | 62.5 (10.3) |
| Sex, No. (%) | |||
| Women | 31 (88.6) | 31 (86.1) | 62 (87.3) |
| Men | 4 (11.4) | 5 (13.9) | 9 (12.7) |
| Race, No. (%) | |||
| White | 27 (77.1) | 28 (77.8) | 55 (77.5) |
| Black/African American | 4 (11.4) | 4 (11.1) | 8 (11.3) |
| Other | 1 (2.9) | 2 (5.6) | 3 (3.0) |
| Not reported | 3 (8.6) | 2 (5.6) | 5 (7.0) |
| Chemotherapy type, No. (%) | |||
| Paclitaxel | 11 (31.4) | 5 (13.9) | 16 (22.5) |
| Oxaliplatin | 2 (5.7) | 2 (5.6) | 4 (5.6) |
| Other taxane | 12 (34.3) | 14 (38.9) | 26 (36.6) |
| Other platinum | 1 (2.9) | 3 (8.3) | 4 (5.6) |
| Both taxane and platinum | 5 (14.3) | 4 (11.1) | 9 (12.7) |
| Other | 4 (11.4) | 8 (22.2) | 12 (16.9) |
| Length of neuropathy, mo (%) | 24.6 (18.0) | 25.9 (18.8) | 25.3 (18.3) |
| Missing, No. | 4 (11.4) | 3 (8.3) | 7 (9.9) |
| Disease features | |||
| Primary disease, No. (%) | |||
| Breast | 27 (77.1) | 25 (69.4) | 52 (73.2) |
| Gastrointestinal | 1 (2.9) | 3 (8.3) | 4 (5.6) |
| Gynecological | 4 (11.4) | 4 (11.1) | 8 (11.3) |
| Other | 3 (8.6) | 4 (11.2) | 7 (9.8) |
| Stage, No. (%) | |||
| 1 or 2 | 21 (60) | 22 (61.1) | 43 (60.6) |
| 3 (no metastatic) | 12 (34.3) | 13 (36.1) | 25 (35.2) |
| Missing | 2 (5.7) | 1 (2.8) | 3 (4.2) |
| Taking medication for CIPN, No. (%)b | |||
| Yes | 10 (28.6) | 11 (30.6) | 21 (29.6) |
| No | 18 (51.4) | 18 (50) | 36 (50.7) |
| Missing | 7 (20) | 7 (19.4) | 14 (19.7) |
Abbreviations: CIPN, chemotherapy-induced peripheral neuropathy; SD, standard deviation.
Range, 41 to 80 y.
Duloxetine, venlafaxine, gabapentin, or pregabalin.
By the end of the treatment period, the NFB group demonstrated a significantly greater decrease in worst pain, our primary outcome (mean change score, −2.43; 95% confidence interval [CI], −3.58 to −1.28), than the control group (mean change score, 0.09; 95% CI, −0.72 to −0.90; P = .001) with an effect size of 0.83.
Similar group differences were found for secondary outcomes, including average pain (NFB, −2.2; 95% CI, −3.22 to −1.17; WLC, 0.13; 95% CI, −0.55 to −0.80; P = .001; effect size, 0.88) and pain interference (NFB, −1.86; 95% CI, −1.01 to 2.71; WLC, −0.02; 95% CI, −0.80 to 0.84; P = .009; effect size, 0.66). Analyses demonstrated statistically significant differences in 3 other subscales of the BPI (Table 2).
TABLE 2.
Baseline and Follow-Up Results for the Primary and Secondary Time Points: Treatment Versus Control
| Measure | Mean (SE)
|
Pa | Effect Sizeb | |||||
|---|---|---|---|---|---|---|---|---|
| Treatment (n = 30)
|
Control (n = 32)
|
|||||||
| Baseline | Follow-Up | Change | Baseline | Follow-Up | Change | |||
| Brief Pain Inventory | ||||||||
| Worst pain | 6.00 (0.40) | 3.57 (0.44) | −2.40 (0.56) | 5.53 (0.45) | 5.62 (0.43) | 0.09 (0.40) | .001 | 0.83 |
| Average pain | 4.90 (0.35) | 2.70 (0.38) | −2.20 (0.50) | 4.38 (0.44) | 4.50 (0.35) | 0.13 (0.33) | .001 | 0.88 |
| Pain interference | 3.35 (0.30) | 1.49 (0.30) | −1.86 (0.42) | 2.92 (0.24) | 2.90 (0.45) | −0.02 (0.40) | .009 | 0.66 |
| Pain severity | 4.70 (0.27) | 2.70 (0.38) | −2.00 (0.46) | 4.58 (0.27) | 4.25 (0.36) | −0.33 (0.38) | .001 | 0.77 |
| Activity interference | 4.15 (0.33) | 1.80 (0.31) | −2.35 (0.46) | 3.71 (0.27) | 3.59 (0.51) | −0.12 (0.48) | .001 | 0.76 |
| Affective interference | 2.74 (0.31) | 1.25 (0.32) | −1.50 (0.42) | 2.33 (0.25) | 2.39 (0.44) | 0.06 (0.39) | .042 | 0.51 |
| PQAS | ||||||||
| Unpleasantness | 6.43 (0.36) | 3.17 (0.43) | −3.27 (0.49) | 6.39 (0.37) | 5.74 (0.40) | −0.65 (0.30) | .000 | 1.17 |
| Numbness | 6.53 (0.51) | 2.90 (0.44) | −3.63 (0.47) | 6.67 (0.52) | 5.68 (0.50) | −1.00 (0.47) | .000 | 1.09 |
| Tingling | 6.19 (0.56) | 2.84 (0.39) | −3.35 (0.49) | 6.61 (0.49) | 5.64 (0.42) | −0.10 (0.39) | .000 | 1.25 |
| Intensity | 5.90 (0.38) | 3.52 (0.40) | −2.39 (0.49) | 5.27 (0.46) | 5.87 (0.39) | −0.60 (0.41) | .001 | 0.73 |
| Tenderness | 4.40 (0.45) | 1.17 (0.26) | −3.20 (0.48) | 3.68 (0.56) | 3.84 (0.50) | 0.16 (0.54) | .000 | 1.19 |
| Surface pain | 4.90 (0.53) | 2.65 (0.40) | −2.25 (0.55) | 5.42 (0.44) | 6.00 (0.41) | −0.55 (0.38) | .000 | 1.33 |
| Deep pain | 4.23 (0.37) | 1.42 (0.22) | −2.81 (0.38) | 3.88 (0.43) | 3.50 (0.42) | −0.38 (0.30) | .000 | 1.10 |
| Sensitive | 3.23 (0.43) | 1.27 (0.25) | −1.96 (0.55) | 3.77 (0.60) | 3.13 (0.54) | −0.65 (0.63) | .004 | 0.78 |
| Itchy | 2.0 (0.49) | 0.73 (0.24) | −1.27 (0.36) | 1.52 (0.46) | 2.06 (0.52) | 0.55 (0.47) | .003 | 0.59 |
| Sharp | 4.35 (0.51) | 2.16 (0.43) | −2.19 (0.54) | 4.25 (0.52) | 4.00 (0.55) | −0.26 (0.32) | .001 | 0.67 |
| Hot | 2.71 (0.44) | 1.00 (0.30) | −1.71 (0.39) | 2.19 (0.55) | 2.16 (0.47) | −0.03 (0.33) | .001 | 0.53 |
| Dull | 4.16 (0.56) | 2.45 (0.35) | −1.71 (0.54) | 4.81 (0.46) | 3.94 (0.44) | −0.87 (0.58) | .017 | 0.67 |
| Cold | 3.64 (0.61) | 1.45 (0.39) | −2.19 (0.50) | 2.58 (0.54) | 3.25 (0.63) | 0.68 (0.37) | .000 | 0.62 |
| Shooting | 3.77 (0.61) | 1.27 (0.35) | −2.50 (0.55) | 3.71 (0.54) | 3.90 (0.56) | 0.19 (0.41) | .000 | 1.02 |
| Electrical | 3.53 (0.62) | 1.67 (0.41) | −1.87 (0.69) | 3.45 (0.62) | 3.77 (0.53) | 0.32 (0.70) | .002 | 0.80 |
| Cramping | 4.71 (0.58) | 1.68 (0.40) | −3.03 (0.57) | 3.70 (0.62) | 3.13 (0.53) | −0.55 (0.52) | .002 | 0.56 |
| Radiating | 4.10 (0.58) | 0.65 (0.22) | −3.45 (0.58) | 3.65 (0.53) | 3.19 (0.55) | −0.45 (0.35) | .000 | 1.10 |
| Throbbing | 3.42 (0.57) | 0.81 (0.28) | −2.61 (0.46) | 3.71 (0.63) | 3.77 (0.60) | 0.06 (0.47) | .000 | 1.13 |
| Aching | 4.55 (0.54) | 0.90 (0.25) | −3.65 (0.48) | 3.68 (0.55) | 3.32 (0.55) | −0.35 (0.56) | .000 | 1.02 |
| Heavy | 4.29 (0.66) | 1.26 (0.37) | −3.03 (0.61) | 3.55 (0.58) | 3.35 (0.60) | −0.19 (0.47) | .000 | 0.75 |
Abbreviations: PQAS, Pain Quality Assessment Scale; SE, standard error.
P values were acquired from a general linear model.
Effect sizes were calculated on the basis of group differences in change scores from the pretraining period to the posttraining period with Cohen’s d.
With the PQAS, group differences were noted for unpleasantness (NFB, −3.27; 95% CI, −4.26 to −2.27; WLC, −0.65; 95% CI, −1.26 to −0.03; P = .000; effect size, 1.17) and 19 other subscales (Table 2). No adverse events were reported.
After treatment, there was a significant group × time interaction in which the NFB group presented with significantly increased α activity (P = .021) and decreased β activity (P = .021); this was not seen in the control group. An increase in the ratio of α to β was seen after NFB (Fig. 1A). There was a significant difference between groups in α relative power at the baseline: α was lower in the treatment group. However, after treatment, the NFB group was able to surpass the WLC group in α relative power (Fig. 1B). There was a decrease in β in both groups; however, the magnitude of the decrease was larger in the NFB group than the WLC group (Fig. 1C).
Figure 1.
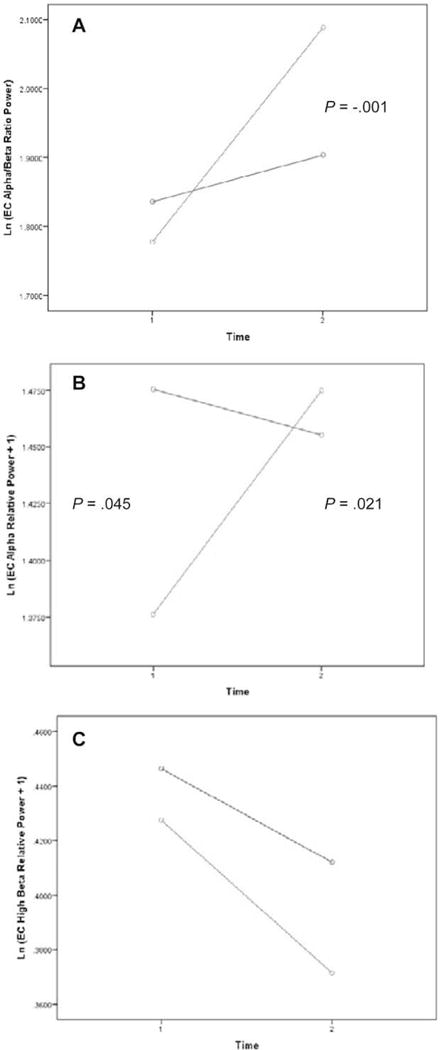
Changes in α and β frequency bands from the period before neurofeedback to the period after neurofeedback: (A) eyes closed, a/b power ratio, Ln(x)-transformed; (B) eyes closed, a relative power, Ln(x + 1)-transformed; and (C) eyes closed, b relative power, Ln(x + 1)-transformed. Green indicates the neurofeedback group; blue indicates the control group. The treatment group was able to increase α while decreasing β (more α, less β).
Results from an analysis of changes in self-reported pain and brain activity revealed significant correlations between decreased worst-pain reporting (BPI) and a reduction of β power (13–45 Hz) in the right (r = −0.39; P = .04) and left parietal cortices (r = −0.38; P = .04) and the frontal (r = −0.47; P = .01), central (r = −0.44; P = .02), and parietal midline regions (r = −0.42; P = .03). There were no significant associations between increases in α (8–12 Hz) activity or the α/β power ratio and worst-pain reporting.
A LORETA analysis demonstrated that in a comparison of the NFB and WLC groups at the end of treatment, both groups showed differences in activity in regions previously thought to contribute to the placebo effect. Specifically, the NFB group showed more activity in the dorsolateral prefrontal cortex (Montreal Neurologic Institute (MNI), −3, 53, 23; t = 2.15) than the WLC group did; however, the WLC group showed more activity in the insula (MNI, 35, −5, −1; t = −1.63) than the NFB group, and neither group showed differences in the rostral anterior cingulate cortex (MNI, 16, 34, 14; Fig. 2).
Figure 2.
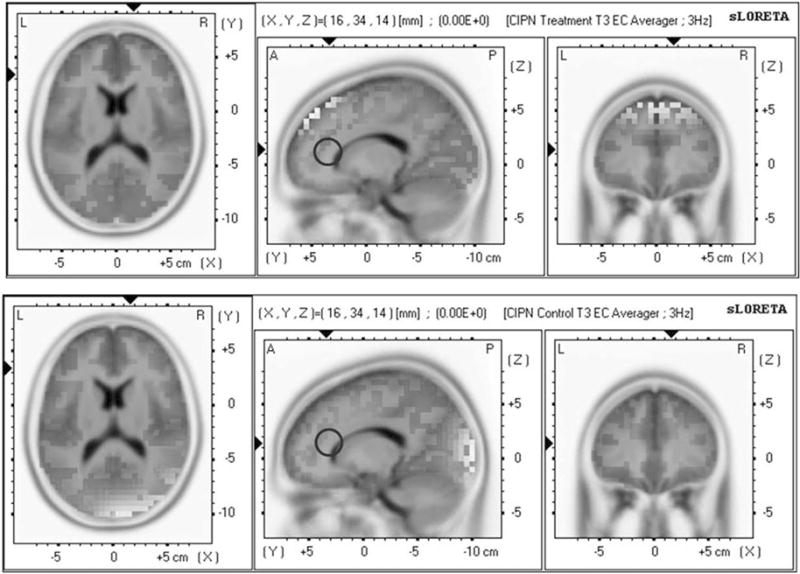
The treatment group (top row) and the control group (bottom row) showed no statistically significant changes in the rostral anterior cingulate, which is important for a placebo response. CIPN indicates chemotherapy-induced peripheral neuropathy; L, left; R, right; sLORETA, standardized low-resolution electromagnetic tomography.
DISCUSSION
To date, this pilot study is the largest clinical trial to determine effects of neuromodulation in cancer survivors. Furthermore, for survivors suffering from CIPN, a common side effect of chemotherapy for which effective treatments remain elusive, we have demonstrated that NFB appears to be an effective symptom-control strategy. For the primary outcome of worst pain, we found statistically and clinically significant reductions for the NFB group versus the WLC group. Importantly, we also saw significant improvements in all domains of secondary outcomes, including nonpainful neuropathic symptoms such as numbness and tingling.
We analyzed changes in brain activity in relation to NFB and in association with pain outcomes. We found statistically significant differences over time and between groups within the frequency bands (α and β) and the scalp locations that we were training. This indicates that NFB training can be specifically directed to particular areas of the cortex and that the cortex responds accordingly, even specifically to the electrical frequency trained. Previous EEG and pain literature suggests that α frequencies are key to a reduction in the severity of symptoms.29 Interestingly, self-reporting of pain was correlated only with a reduction of fast wave activity (β) and was not associated with increases in α or in the ratio of α to β. We conclude that NFB is a targeted intervention with measureable brain and behavioral outcomes.
As of June 14, 2016, we found 269 randomized controlled trials; however, none of these trials showed the efficacy of complementary modalities in treating CIPN, although a few showed incidental improvements.30 Although there are many neuroleptic agents that have demonstrated efficacy in neuropathic pain due to other etiologies such as diabetes (gabapentin and pregabalin), duloxetine is the only pharmaceutical that has shown a significant benefit for CIPN and is the only agent recommended by American Society of Clinical Oncology guidelines31; otherwise, there is no consistent evidence supporting any pharmaceutical’s efficacy in treating CIPN symptoms. In the registration trial of duloxetine for CIPN, which also examined effects in any cancer type and after paclitaxel, taxane, or oxaliplatin treatment, the mean reduction in the average-pain ratings was 1.06 for duloxetine and 0.34 for a placebo with a moderate effect size of 0.51.1 However, the use of duloxetine was associated with fatigue and nausea and had a dropout rate of 12%.1 In our study, the mean decrease in average pain with NFB was 2.2 points with an effect size of 0.88. We did not note any negative side effects, and patients attended 100% of the treatment sessions.
One limitation of the current study is that we did not have a placebo group. Because of this, we analyzed regions of the brain that are shown to be active in placebo analgesia, including regions of the brain that are associated with patient-reported outcomes during placebo conditions.32 Our results suggest that although the placebo effect may be a factor in this study, it was not the only factor leading to improvements in symptoms. Also, although the brain analyses used a Bonferroni correction, the results should still be considered exploratory and should be interpreted with caution.
Another limitation is that most of our participants were female and breast cancer survivors, so future research should investigate the efficacy of NFB by chemotherapy type. An investigation is also needed that includes a sham NFB intervention to elucidate the exact mechanisms of NFB. Other questions to investigate include the role of NFB in the prevention of CIPN and other cancer pain conditions, the effectiveness of NFB in acute pain settings, and the role of NFB in symptom management both during and after active cancer treatment. Lastly, medications given for CIPN could affect the EEG; however, our participants had symptoms even though they may have been on medications, and there were no differences between the 2 groups in the number of participants taking CIPN medications at the baseline.
Because of the prevalence of CIPN in the cancer survivorship population and the dearth of effective therapies, the clinical implications of our findings are readily apparent. NFB is safe, portable, complementary to other treatment regimens, and relatively inexpensive. EEG NFB appears to be effective at reducing CIPN symptoms and could potentially treat neuropathy due to other conditions; especially because pharmaceuticals that treat CIPN may have side effects of their own, it is important to consider treatments that minimize or eliminate additional adverse effects for especially vulnerable populations with already existing comorbidities.
Acknowledgments
We acknowledge Stephanie Gabel-Zepeda, PhD (The University of Texas MD Anderson Cancer Center), for her help with data collection and NovaTech EEG for its help with data analysis. We also thank the participants for their contributions to the trial.
FUNDING SUPPORT
This study was funded by the American Cancer Society (121297-PF-11-169-01-PCSM), the Rising Tide Foundation, the Hille Foundation, and the National Center for Complementary and Integrative Health (1K01AT008485-01).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Cathy Eng reports receiving grants from Daiichi and Keryx, participating in a speakers’ bureau for Genentech, and receiving honoraria from Roche/Genentech and Bayer outside the submitted work.
AUTHOR CONTRIBUTIONS
Sarah Prinsloo: Principal investigator, concept and design, data analysis, manuscript writing, and final approval of the manuscript. Diane Novy: Participant referrals to the trial, manuscript writing, and final approval of the manuscript. Larry Driver: Participant referrals to the trial, manuscript writing, and final approval of the manuscript. Randall Lyle: Concept and design, manuscript writing, data interpretation, and final approval of the manuscript. Lois Ramondetta: Participant referrals to the trial, manuscript writing, and final approval of the manuscript. Cathy Eng: Participant referrals to the trial, manuscript writing, and final approval of the manuscript. Jennifer McQuade: Data interpretation, manuscript writing, and final approval of the manuscript. Gabriel Lopez: Participant referrals to the trial, manuscript writing, and final approval of the manuscript. Lorenzo Cohen: Concept and design, manuscript writing, data interpretation, and final approval of the manuscript.
References
- 1.Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309:1359–1367. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:1941–1967. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 3.Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155:2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Siegal T, Haim N. Cisplatin-induced peripheral neuropathy. Frequent off-therapy deterioration, demyelinating syndromes, and muscle cramps. Cancer. 1990;66:1117–1123. doi: 10.1002/1097-0142(19900915)66:6<1117::aid-cncr2820660607>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Loprinzi CL, Reeves BN, Dakhil SR, et al. Natural history of paclitaxel-associated acute pain syndrome: prospective cohort study NCCTG N08C1. J Clin Oncol. 2011;29:1472–1478. doi: 10.1200/JCO.2010.33.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majithia N, Temkin SM, Ruddy KJ, Beutler AS, Hershman DL, Loprinzi CL. National Cancer Institute–supported chemotherapy-induced peripheral neuropathy trials: outcomes and lessons. Support Care Cancer. 2016;24:1439–1447. doi: 10.1007/s00520-015-3063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao RD, Michalak JC, Sloan JA, et al. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3) Cancer. 2007;110:2110–2118. doi: 10.1002/cncr.23008. [DOI] [PubMed] [Google Scholar]
- 8.Stubblefield MD, Burstein HJ, Burton AW, et al. NCCN task force report: management of neuropathy in cancer. J Natl Compr Canc Netw. 2009;7(suppl 5):S1–S26. doi: 10.6004/jnccn.2009.0078. [DOI] [PubMed] [Google Scholar]
- 9.Sharpe L, Ianiello M, Dear BF, Nicholson Perry K, Refshauge K, Nicholas MK. Is there a potential role for attention bias modification in pain patients? Results of 2 randomised, controlled trials. Pain. 2012;153:722–731. doi: 10.1016/j.pain.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Del Casale A, Ferracuti S, Rapinesi C, et al. Hypnosis and pain perception: an activation likelihood estimation (ALE) meta-analysis of functional neuroimaging studies. J Physiol Paris. 2015;109:165–172. doi: 10.1016/j.jphysparis.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Leibovici V, Magora F, Cohen S, Ingber A. Effects of virtual reality immersion and audiovisual distraction techniques for patients with pruritus. Pain Res Manage. 2009;14:283–286. doi: 10.1155/2009/178751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.deCharms RC, Maeda F, Glover GH, et al. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci U S A. 2005;102:18626–18631. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bray S, Shimojo S, O’Doherty JP. Direct instrumental conditioning of neural activity using functional magnetic resonance imaging-derived reward feedback. J Neurosci. 2007;27:7498–7507. doi: 10.1523/JNEUROSCI.2118-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caria A, Sitaram R, Veit R, Begliomini C, Birbaumer N. Volitional control of anterior insula activity modulates the response to aversive stimuli. A real-time functional magnetic resonance imaging study. Biol Psychiatry. 2010;68:425–432. doi: 10.1016/j.biopsych.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Caro XJ, Winter EF. EEG biofeedback treatment improves certain attention and somatic symptoms in fibromyalgia: a pilot study. Appl Psychophysiol Biofeedback. 2011;36:193–200. doi: 10.1007/s10484-011-9159-9. [DOI] [PubMed] [Google Scholar]
- 16.deCharms RC. Applications of real-time fMRI. Nat Rev Neurosci. 2008;9:720–729. doi: 10.1038/nrn2414. [DOI] [PubMed] [Google Scholar]
- 17.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 18.Atkinson TM, Mendoza TR, Sit L, et al. The Brief Pain Inventory and its “pain at its worst in the last 24 hours” item: clinical trial endpoint considerations. Pain Med. 2010;11:337–346. doi: 10.1111/j.1526-4637.2009.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen MP, Gammaitoni AR, Olaleye DO, Oleka N, Nalamachu SR, Galer BS. The Pain Quality Assessment Scale: assessment of pain quality in carpal tunnel syndrome. J Pain. 2006;7:823–832. doi: 10.1016/j.jpain.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol. 2002;24(suppl D):5–12. [PubMed] [Google Scholar]
- 21.Mulert C, Jager L, Schmitt R, et al. Integration of fMRI and simultaneous EEG: towards a comprehensive understanding of localization and time-course of brain activity in target detection. Neuroimage. 2004;22:83–94. doi: 10.1016/j.neuroimage.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 22.Vitacco D, Brandeis D, Pascual-Marqui R, Martin E. Correspondence of event-related potential tomography and functional magnetic resonance imaging during language processing. Hum Brain Mapp. 2002;17:4–12. doi: 10.1002/hbm.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dierks T, Jelic V, Pascual-Marqui RD, et al. Spatial pattern of cerebral glucose metabolism (PET) correlates with localization of intrace-rebral EEG-generators in Alzheimer’s disease. Clin Neurophysiol. 2000;111:1817–1824. doi: 10.1016/s1388-2457(00)00427-2. [DOI] [PubMed] [Google Scholar]
- 24.Thatcher RW, North DM, Biver CJ. Diffusion spectral imaging modules correlate with EEG LORETA neuroimaging modules. Hum Brain Mapp. 2012;33:1062–1075. doi: 10.1002/hbm.21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Field A. Discovering Statistics Using IBM SPSS statistics. 4th. London, United Kingdom: Sage Publications Ltd; 2013. [Google Scholar]
- 26.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Vol. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 27.Stevens J. Applied Multivariate Statistics for the Social Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1986. [Google Scholar]
- 28.Nuwer MR. Quantitative EEG: I. Techniques and problems of frequency analysis and topographic mapping. J Clin Neurophysiol. 1988;5:1–43. [PubMed] [Google Scholar]
- 29.Kayiran S, Dursun E, Dursun N, Ermutlu N, Karamursel S. Neuro-feedback intervention in fibromyalgia syndrome; a randomized, controlled, rater blind clinical trial. Appl Psychophysiol Biofeedback. 2010;35:293–302. doi: 10.1007/s10484-010-9135-9. [DOI] [PubMed] [Google Scholar]
- 30.Brami C, Bao T, Deng G. Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: a systematic review. Crit Rev Oncol Hematol. 2016;98:325–334. doi: 10.1016/j.critrevonc.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical prac-tice guideline. J Clin Oncol. 2014;32:1941–1967. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 32.Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci. 2015;16:403–418. doi: 10.1038/nrn3976. [DOI] [PMC free article] [PubMed] [Google Scholar]


