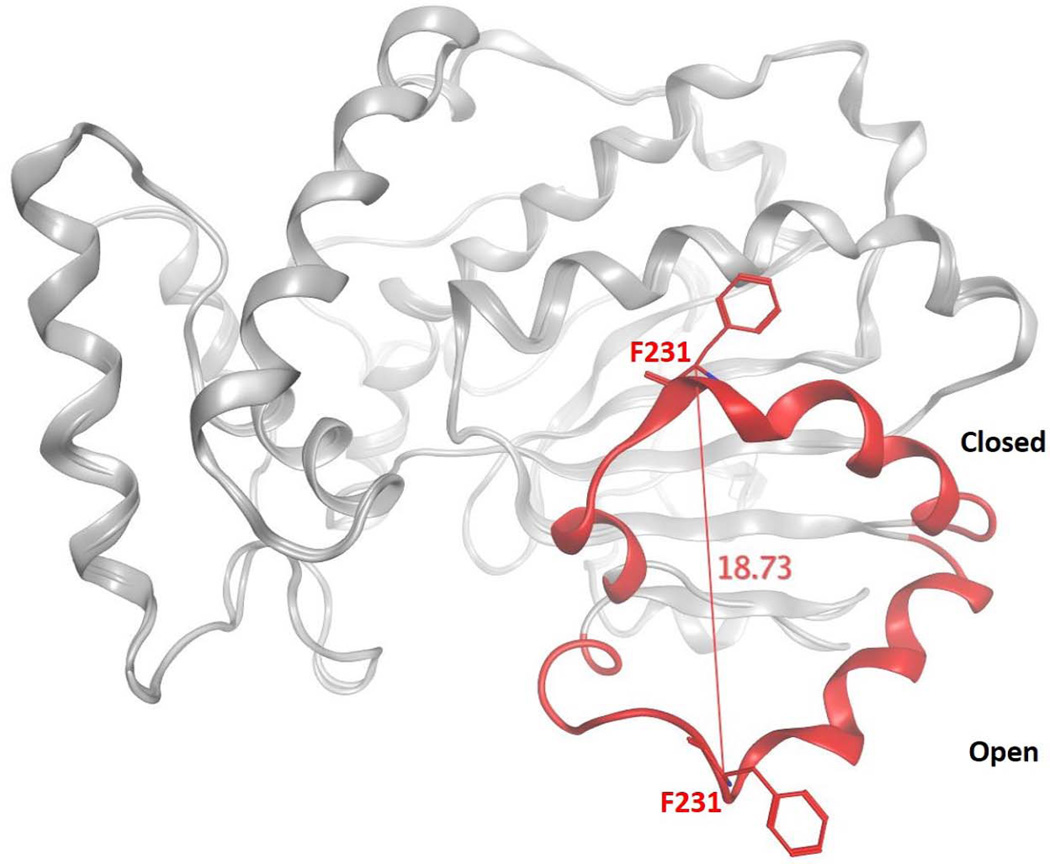
Figure 2.
Conformational transitions of Sec14 during lipid exchange. The ‘open’ and ‘closed’ conformers of Se14 are superimposed to highlight the motion of the gating helix that controls access to the Sec14 hydrophobic pocket. The Sec14 α-carbon backbone is represented in gray ribbon with the helical gate structural element rendered in red color. Distance monitoring of the Cα atom of residue F231 of the gate illustrates the 18 Å displacement of the helical gate.