Abstract
During a survey of saprophytic microfungi on decomposing woody, herbaceous debris and soil from different regions in Southern Europe, a wide range of interesting species of asexual ascomycetes were found. Phylogenetic analyses based on partial gene sequences of SSU, LSU and ITS proved that most of these fungi were related to Sordariomycetes and Dothideomycetes and to lesser extent to Leotiomycetes and Eurotiomycetes. Four new monotypic orders with their respective families are proposed here, i.e. Lauriomycetales, Lauriomycetaceae; Parasympodiellales, Parasympodiellaceae; Vermiculariopsiellales, Vermiculariopsiellaceae and Xenospadicoidales, Xenospadicoidaceae. One new order and three families are introduced here to accommodate orphan taxa, viz. Kirschsteiniotheliales, Castanediellaceae, Leptodontidiaceae and Pleomonodictydaceae. Furthermore, Bloxamiaceae is validated. Based on morphology and phylogenetic affinities Diplococcium singulare, Trichocladium opacum and Spadicoides atra are moved to the new genera Paradiplococcium, Pleotrichocladium and Xenospadicoides, respectively. Helicoon fuscosporum is accommodated in the genus Magnohelicospora. Other novel genera include Neoascotaiwania with the type species N. terrestris sp. nov., and N. limnetica comb. nov. previously accommodated in Ascotaiwania; Pleomonodictys with P. descalsii sp. nov. as type species, and P. capensis comb. nov. previously accommodated in Monodictys; Anapleurothecium typified by A. botulisporum sp. nov., a fungus morphologically similar to Pleurothecium but phylogenetically distant; Fuscosclera typified by F. lignicola sp. nov., a meristematic fungus related to Leotiomycetes; Pseudodiplococcium typified by P. ibericum sp. nov. to accommodate an isolate previously identified as Diplococcium pulneyense; Xyladictyochaeta typified with X. lusitanica sp. nov., a foliicolous fungus related to Xylariales and similar to Dictyochaeta, but distinguished by polyphialidic conidiogenous cells produced on setiform conidiophores. Other novel species proposed are Brachysporiella navarrica, Catenulostroma lignicola, Cirrenalia iberica, Conioscypha pleiomorpha, Leptodontidium aureum, Pirozynskiella laurisilvatica, Parasympodiella lauri and Zanclospora iberica. To fix the application of some fungal names, lectotypes and/or epitypes are designated for Magnohelicospora iberica, Sporidesmium trigonellum, Sporidesmium opacum, Sporidesmium asperum, Camposporium aquaticum and Psilonia atra.
Key words: Biodiversity, Dothideomycetes, Eurotiomycetes, Leotiomycetes, Sordariomycetes, Systematics
Taxonomical novelties: New orders: Kirschsteiniotheliales Hern.-Restr., Gené, R.F. Castañeda & Crous; Lauriomycetales Hern.-Restr., R.F. Castañeda & Guarro; Parasympodiellales Hern.-Restr., Gené, R.F. Castañeda & Crous; Vermiculariopsiellales Hern.-Restr., J. Mena, Gené & Crous; Xenospadicoidales Hern.-Restr., J. Mena & Gené
New families: Castanediellaceae Hern.-Restr., Guarro & Crous; Lauriomycetaceae Hern.-Restr., R.F. Castañeda & Guarro; Leptodontidiaceae Hern.-Restr., Crous & Gené; Parasympodiellaceae Hern.-Restr., Gené, Guarro & Crous; Pleomonodictydaceae Hern.-Restr., J. Mena & Gené; Vermiculariopsiellaceae Hern.-Restr., J. Mena, Gené & Crous; Xenospadicoidaceae Hern.-Restr., J. Mena & Gené
New genera: Anapleurothecium Hern.-Restr., R.F. Castañeda & Gené; Fuscosclera Hern.-Restr., J. Mena & Gené; Neoascotaiwania Hern.-Restr., R.F. Castañeda & Guarro; Paradiplococcium Hern.-Restr., J. Mena & Gené; Pleomonodictys Hern.-Restr., J. Mena & Gené; Pleotrichocladium Hern.-Restr., R.F. Castañeda & Gené; Pseudodiplococcium Hern.-Restr., J. Mena & Gené; Xenospadicoides Hern.-Restr., J. Mena & Gené; Xyladictyochaeta Hern.-Restr., R.F. Castañeda & Gené
New species: Anapleurothecium botulisporum Hern.-Restr., R.F. Castañeda & Gené; Brachysporiella navarrica Hern.-Restr., R.F. Castañeda & Gené; Catenulostroma lignicola Hern.-Restr., J. Mena & Gené; Cirrenalia iberica Hern.-Restr. & Gené; Conioscypha pleiomorpha Hern.-Restr., R.F. Castañeda & Gené; Fuscosclera lignicola Hern.-Restr., J. Mena & Gené; Leptodontidium aureum Hern.-Restr., Guarro & Gené; Parasympodiella lauri Hern.-Restr., Gene & Guarro; Parasympodiella lauri Hern.-Restr., Gene & Guarro; Pirozynskiella laurisilvatica Hern.-Restr., R.F. Castañeda & Gené; Pleomonodictys descalsii Hern.-Restr., J. Mena & Gené; Pseudodiplococcium ibericum Hern.-Restr., J. Mena & Gené; Xyladictyochaeta lusitanica Hern.-Restr., R.F. Castañeda & Gené; Zanclospora iberica Hern.-Restr., J. Mena & Gené
New combinations: Magnohelicospora fuscospora (Linder) R.F. Castañeda, Hern.-Restr. & Gené; Neoascotaiwania limnetica (H.S. Chang & S.Y. Hsieh) Hern.-Restr., R.F. Castañeda & Gené; Paradiplococcium singulare (Hern.-Restr., J. Mena, Gené & Guarro) Hern.-Restr., J. Mena & Gené; Pleomonodictys capensis (R.C. Sinclair, Boshoff & Eicker) Hern.-Restr., J. Mena & Gené; Pleotrichocladium opacum (Corda) Hern.-Restr., R.F. Castañeda & Gené; Xenospadicoides atra (Corda) Hern.-Restr., J. Mena & Gené
Typifications: Lectotypifications: Camposporium aquaticum Dudka, Psilonia atra Corda, Sporidesmium asperum Corda, Sporidesmium opacum Corda
Epitypifications: Magnohelicospora iberica R.F. Castañeda, Hern.-Restr., Gené & Guarro; Sporidesmium trigonellum Sacc.; Sporidesmium opacum Corda; Sporidesmium asperum Corda; Camposporium aquatium Dudka; Psilonia atra Corda
Introduction
Fungi are hyper-diverse organisms, and although only 100 000 species are presently acknowledged, species numbers are estimated to range between 1.5 to 5.1 million (Hawksworth, 2004, Blackwell, 2011). This high diversity is partly due to the fact that many fungi are cosmopolitan, having a wider geographical distribution than plants and other organisms. Furthermore, many habitats and substrates remain unexplored as far as Fungi are concerned, and potentially might support many undescribed species. In addition, the use of new isolation techniques, culture media and molecular, DNA-based data will reveal many of the species that have thus far been overlooked (Hawksworth & Rossman 1997).
The current classification of the kingdom Fungi is based largely on polyphasic taxonomy in which numerous authors have attempted to integrate morphological and molecular data (Huhndorf et al., 2004, Geiser et al., 2006, James et al., 2006, Spatafora et al., 2006, Wang et al., 2006, Hibbett et al., 2007, McLaughlin et al., 2009, Schoch et al., 2009, Hyde et al., 2013, Liu et al., 2015a, Liu et al., 2015b, Wang et al., 2015a, Wang et al., 2015b, Wang et al., 2015c, Vu et al., 2016). DNA barcoding based on the internal transcribed spacer (ITS) region has become, among several other molecular techniques, an important tool for species identification (Quaedvlieg et al., 2012, Schoch et al., 2012). However, the ITS is inconclusive in some genera and, therefore, additional genes such as, LSU, tef1, tub, rpb2, etc. are required for a more accurate identification (Stielow et al. 2015). Nevertheless, the majority of the described fungal species are only represented by dried specimens in fungaria and lack DNA barcode data (Crous et al., 2014a, Crous et al., 2015a), which represents a significant handicap for defining either a phylogenetic species concept, or in the best case, an integrated or consolidated species concept. Therefore, there is an important need for field studies in order to recollect and hopefully to isolate in pure culture as many fungal species as possible what would allow proper morphological and molecular characterisation. Furthermore, when necessary, this approach will also allow for the re-typification of taxa with living cultures and DNA barcodes.
In this context, different surveys were conducted in several areas of ecological interest of the Iberian continental and insular (Baleares and Canary Islands) areas in order to explore the diversity of microfungi in various substrates including litter, submerged dead plant material and soil (Mena-Portales et al., 2011, Mena-Portales et al., 2015, Mena-Portales et al., 2016, Hernández-Restrepo et al., 2012, Hernández-Restrepo et al., 2013, Hernández-Restrepo et al., 2014a, Hernández-Restrepo et al., 2014b, Castañeda-Ruiz et al., 2012, Madrid et al., 2016). As initial approach, we tried to culture and identify all of these fungi, and to elucidate their phylogeny within the Ascomycota.
In the present study, the taxonomy of more than 50 fungi, including 14 new species and nine new genera, has been resolved. Based on the combination of morphological features and sequence analyses of the nuclear rDNA operon, five new orders and seven new families distributed in different classes (i.e. Dothideomycetes, Eurotiomycetes, Leotiomycetes and Sordariomycetes) are introduced. Living cultures and sequences of the taxa found in the above-mentioned surveys have been deposited in public culture collections and DNA sequence databases, respectively.
Materials and methods
Isolates
The microfungi were isolated from dead leaves, wood, bark, seeds and soil samples collected in several natural areas of the Iberian Peninsula (Spain and Portugal) and Islands, during a period from 2009 to 2013, mainly during spring and autumn (Table 1). Plant debris were placed in moist chambers and treated according to Castañeda-Ruiz et al. (2016). Fungi from soil samples were isolated by using wood baiting and dilution-plating techniques, following the techniques described in Calduch et al. (2004). Single-conidial cultures were performed on water agar (Difco agar 5 g, 1 000 mL tap water, pH 6). All the isolates are maintained in the culture collection of the Faculty of Medicine at the Rovira i Virgili University (FMR), Reus, Spain. Type specimens and ex-type cultures of the novel fungi were deposited in the Westerdijk Fungal Biodiversity Institute (CBS), Utrecht, the Netherlands (Table 1), and some also in the Belgian Co-ordinated Collections of Microorganisms (MUCL), Belgium, and in Kew Royal Botanical Gardens, England. Additional type specimens, ex-type cultures or strains of different species used for comparison were obtained from the CBS culture collection (Table 1). The identification of the reference strains included in the study was confirmed mainly based on the comparison with the protologues and/or examination of holotypes when possible. Nomenclatural novelties and descriptions were deposited in MycoBank (Crous et al. 2004).
Table 1.
List of isolates included in the study.
| Taxa1 | Preliminary Identification | Strain2 | Substrate | Locality3 | Fungal Class | GenBank4 |
||
|---|---|---|---|---|---|---|---|---|
| SSU | LSU | ITS | ||||||
| Anapleurothecium botulisporum gen. et sp. nov.* | FMR 11490, CBS 132713 | Dead wood | Spain, Asturias, Picos de Europa N.P. | Sordariomycetes | KY853483 | KY853423 | ||
| Anungitea syzygii* | FMR 11934 | Dead wood | Spain, Castilla-La Mancha, Hayedo de la Tejera Negra | Dothideomycetes | KY853484 | KY853424 | ||
| Bactrodesmiastrum moniliodes† | FMR 10756, CBS 137251 | Dead wood | Spain, Aragón, Ordesa y Monte Perdido N.P. | Sordariomycetes | KF771879 | KF771878 | ||
| FMR 11337, CBS 137252 | Dead wood | Spain, Asturias, Picos de Europa N.P. | Sordariomycetes | KF771877 | KF771876 | |||
| B.obovatum† | Janetia obovata | FMR 6482, CBS 101300 | Dead wood | Spain, Mallorca, Sierra de Tramuntana | Sordariomycetes | FR870266 | FR870264 | |
| B.pyriforme† | FMR 10747, CBS 127867 | Dead wood | Spain, Aragón, Ordesa y Monte Perdido N.P. | Sordariomycetes | FR870263 | FR870265 | ||
| Bactrodesmium pallidum* | FMR 11345, CBS 130515 | Dead wood | Spain, Cantabria, Saja-Besaya N.P. | Sordariomycetes | KY853485 | KY853425 | ||
| Bloxamia truncata* | FMR 11240 | Dead wood | Spain, Cantabria, Saja-Besaya N.P. | Leotiomycetes | KY853486 | KY853426 | ||
| Brachysporiella navarrica sp. nov. | Brachysporiella sp. | FMR 12426, CBS 142296 | Dead wood | Spain, Navarra, Baribar | Dothideomycetes | HF937359 | KY853487 | KY853427 |
| Cacumisporium capitulatum | Chaetosphaeria decastyla | FMR 11339 | Dead wood | Spain, Galicia, Las Fragas del Eume P. | Sordariomycetes | HF677190 | HF677176 | |
| Camposporium antennatum | “Paradendryphiella salina” | CBS 734.96 | Unknown | Cuba | Dothideomycetes | KF156156 | KF156100 | |
| C.cambrense* | FMR 12069 | Submerged wood | Spain, Aragón, Sierra y Cañones del Guara P. | Dothideomycetes | HF937343 | KY853488 | KY853428 | |
| Camposporium sp. | MHR 1565 | Dead wood | Thailand, Nan province, Bo Kluea | Dothideomycetes | MF155650 | |||
| Catenulostroma lignicola sp. nov. | FMR 11491, CBS 130285 | Dead wood | Spain, Galicia, Las Fragas del Eume P. | Dothideomycetes | HF937354 | KY853489 | KY853429 | |
| Ceratocladium polysetosum†* | FMR 10750 | Bark | Spain, Aragón, Ordesa y Monte Perdido N.P. | Dothideomycetes | HF937345 | KY853490 | KY853430 | |
| Ceratosporella novae-zealandiae* | FMR 10760 | Dead wood | Spain, Aragón, Teruel | Eurotiomycetes | HF937346 | KY853491 | KY853431 | |
| Chaetopsina fulva | FMR 13129, CBS 137301 | Dead leaves | Spain, Canary Island, La Gomera | Sordariomycetes | KY853492 | KY853432 | ||
| C.penicillata | FMR 10948 | Submerged wood | Spain, Valencia | Sordariomycetes | KY853493 | KY853433 | ||
| Chalara hughesii | FMR 12413, CBS 142292 | Dead wood | Spain, Navarra, Baribar | Leotiomycetes | KY853494 | KY853434 | ||
| Chloridium chloroconium | Gonytrichum chlamydosporoides var. simile | FMR 11940 | Dead wood of Quercus | Spain, Burgos, Sierra de la Demanda | Sordariomycetes | KY853495 | KY853435 | |
| Cirrenalia iberica sp. nov. | Cirrenalia sp. | FMR 12149, CBS 142289 | Soil | Spain, Aragón, Ordesa y Monte Perdido N.P. | Sordariomycetes | KY853496 | KY853436 | |
| FMR 12418, CBS 142295 | Submerged wood | Spain, Aragón, Valles Occidentales P. | Sordariomycetes | HF678542 | HF678532 | |||
| Cladophialophora pseudocarrionii† | FMR 12062, CBS 138591 | Soil | Spain, Castilla-La Mancha, Hayedo de la Tejera Negra | Eurotiomycetes | KU705844 | KU705827 | ||
| Conioscypha hoehnelii* | FMR 11592 | Dead wood | Spain, Castilla y León, San Pedro de Arlanza | Sordariomycetes | HF937348 | KY853497 | KY853437 | |
| C.minutispora† | FMR 11245, CBS 137253 | Twig | Spain, Cantabria, Saja-Besaya N.P | Sordariomycetes | KF924559 | KF924559 | ||
| C.pleiomorpha sp. nov. | Conioscypha sp. | FMR 13134, CBS 138110 | Dead wood | Spain, Canary Islands, Tenerife, Las Mercedes | Sordariomycetes | KY853498 | KY853438 | |
| Cordana verruculosa† | FMR 10754, CBS 121870 | Dead wood | Spain, Aragón, Ordesa y Monte Perdido N.P. | Sordariomycetes | HE672163 | HE716752 | ||
| Dematioscypha dematiicola | Haplographium delicatum | FMR 11585 | Dead wood | Spain, Aragón, Ordesa y Monte Perdido N.P. | Leotiomycetes | HF937353 | HF677187 | HF677177 |
| Dictyochaeta fuegiana | Chaetosphaeria fuegiana | FMR 13126 | Dead wood | Spain, Canary Island, La Palma | Sordariomycetes | KY853500 | KY853440 | |
| Dictyosporium elegans | FMR 13125, CBS 137303 | Dead wood | Spain, Asturias, Cangas de Narcea | Dothideomycetes | KY853501 | KY853441 | ||
| Endophragmiella dimorphospora* | FMR 12150 | Soil | Spain, Canary Islands, Barranco Laurisilva | Sordariomycetes | HF937351 | KY853502 | KY853442 | |
| Exophiala equina | FMR 12091 | Soil | Spain, Aragón, Ordesa y Monte Perdido N.P. | Eurotiomycetes | KY853503 | KY853443 | ||
| Fuscosclera lignicola gen. et sp. nov. | FMR 11236, CBS 142287 | Dead wood | Spain, Galicia, Los Ancares P. | Leotiomycetes | KY853504 | KY853444 | ||
| Hansfordia pulvinata* | FMR 12706, CBS 142297 | Grass leaves | Spain, Mallorca, Sierra de Tramuntana | Sordariomycetes | HF937352 | HF678545 | HF678535 | |
| Hyaloscypha aureliella | Cheiromycella microscopica | FMR 10851 | Dead wood | Spain, Aragón, Teruel | Leotiomycetes | KY853505 | KY853445 | |
| FMR 11559 | Dead wood of Pinus sp. | Spain, Castilla y León, Burgos, Hontoria del Pinar | Leotiomycetes | KY853506 | KY853446 | |||
| Jalapriya toruloides | Dictyosporium toruloides | FMR 11942 | Dead wood | Spain, Aragón, Ordesa y Monte Perdido N.P. | Dothideomycetes | HF677188 | HF677181 | |
| FMR 12419 | Dead wood | Spain, Aragón, Valles Occidentales P. | Dothideomycetes | HF678543 | HF678533 | |||
| Lauriomyces bellulus | FMR 12188 | Dead leaves | Portugal, Viana do Castello, Lagoas de Bertiandos, P.A. | Leotiomycetes | HF678541 | HF678531 | ||
| Leptodontidium aureum sp. nov. | FMR 11834, CBS 142316 | Soil | Spain, Galicia, Las Fragas del Eume P. | Leotiomycetes | HF937355 | KY853507 | KY853447 | |
| L.irregulare | CBS 851.73 | Soil | Sweden, Skåne | Leotiomycetes | AY129281 | KY853508 | KY853448 | |
| L.trabinellum | Leptodontidium elatius | CBS 329.53 | Decaying wood of Betula | France | Leotiomycetes | AY129280 | KY853509 | AY129285 |
| Magnohelicospora iberica†* | FMR 12414, CBS 142293 | Dead leaves | Spain, Navarra, Robledal de Orgi | Dothideomycetes | KY853510 | |||
| Menispora glauca* | FMR 12089 | Bark | Spain, Burgos, Sierra de la Demanda | Sordariomycetes | HF678538 | HF678528 | ||
| Monochaetia kansensis | FMR 11156 | Dead wood | Spain, Cantabria, Saja-Besaya N.P. | Sordariomycetes | KY853511 | |||
| Monodictys nigrospermum* | Monodictys levis | FMR 11941, CBS 132489 | Soil | Spain, Galicia, Los Ancares P. | Sordariomycetes | HF677186 | HF677180 | |
| Myrmecridium schulzeri | FMR 12424 | Grass leaves | Spain, Navarra, Robledal de Orgi | Sordariomycetes | KY853512 | KY853451 | ||
| Neoascotaiwania limnetica comb. nov. | Ascotaiwania lignicola | CBS 126576 | Submerged wood of Alnus glutinosa | France, Ariège, Rimont, Peyrau brook | Sordariomycetes | KY853513 | KY853452 | |
| CBS 126792 | Submerged wood | France, Ariège, Rimont, Peyrau brook | Sordariomycetes | KY853514 | KY853453 | |||
| N.terrestris gen. et sp. nov. | FMR 12412, CBS 142291 | Soil | Spain, Asturias, Picos de Europa N.P. | Sordariomycetes | KY853547 | KY853515 | KY853454 | |
| Oncopodiella trigonella* | FMR 10788, CBS 126413 | Bark | Spain, Aragón, Teruel | Dothideomycetes | KY853548 | KY853516 | KY853455 | |
| Paradiplococcium singulare†* gen. et comb. nov. | Diplococcium singulare | FMR 10752, CBS 126091 | Dead wood | Spain, Aragón, Ordesa y Monte Perdido N.P. | Sordariomycetes | KY853517 | KY853456 | |
| Paramyrothecium roridum | Myrothecium roridum | FMR 11946 | Soil | Spain, Aragón, Teruel | Sordariomycetes | HF678539 | HF678529 | |
| Parasympodiella lauri sp. nov. | Parasympodiella sp. | FMR 13132, CBS 138108 | Dead leaves of Laurus sp. | Spain, Canary Islands, La Palma | Sordariomycetes | KY853518 | KY853457 | |
| Phaeodactylium stadleri†* | FMR 12185, CBS 132715 | Dead leaves of Ammophila arenaria | Portugal, Playa de Ofir | Dothideomycetes | HF678536 | HF678526 | ||
| Phaeoisaria sparsa* | FMR 11939 | Dead wood | Spain, Aragón, Ordesa y Monte Perdido N.P. | Sordariomycetes | HF677185 | HF677179 | ||
| Phragmocephala glanduliformis* | FMR 11237 | Bark | Spain, Valencia | Dothideomycetes | HF937357 | KY853519 | KY853458 | |
| Pirozynskiella laurisilvatica sp. nov. | Pirozynskiella sp. | FMR 13133, CBS 138109 | Dead leaves of Laurus sp. | Spain, Canary Islands, La Gomera | Dothideomycetes | KY853520 | KY853459 | |
| Pleomonodictys capensis comb. nov. | Monodictys capensis | CBS 968.97 | South Africa, Western Cape Region | Dothideomycetes | KY853521 | KY853460 | ||
| P.descalsii gen. et sp. nov. | Monodictys sp. | FMR 12716, CBS 142298 | Bark Quercus | Spain, Mallorca, Sierra de Tramuntana | Dothideomycetes | KY853522 | KY853461 | |
| Pleotrichocladium opacum gen. et comb. nov. | Trichocladium opacum | FMR 12088, CBS 142288 | Soil | Spain, Aragón, Ordesa y Monte Perdido N.P. | Dothideomycetes | HF678540 | HF678530 | |
| FMR 12416, CBS 142294 | Dead wood | Spain, Navarra, Robledal de Orgi | Dothideomycetes | KY853523 | KY853462 | |||
| CBS 450.70 | Dead wood of Thuja occidentalis | The Netherlands, Baarn, garden Eemnesserweg 90 | Dothideomycetes | KY853524 | KY853463 | |||
| CBS 534.66 | Soil | Austria, Vorarlberg | Dothideomycetes | KY853525 | KY853464 | |||
| CBS 709.92 | Lichen | Antarctica, King George, Jubany | Dothideomycetes | KY853526 | ||||
| Pseudodiplococcium ibericum* gen. et sp. nov. | “Diplococcium pulneyense” | FMR 10959, CBS 127864 | Dead wood | Spain, Galicia, Los Ancares P. | Sordariomycetes | HF937350 | KY853527 | KY853465 |
| Rhinocladiella amoena† | FMR 12063, CBS 138590 | Submerged wood | Spain, Aragón, Ordesa y Monte Perdido N.P. | Eurotiomycetes | KU705857 | KU705840 | ||
| Solicorynespora insolita† | FMR 11497, CBS 131273 | On bark | Spain, Cantabria, Picos de Europa N.P. | Dothideomycetes | HF677183 | HF677174 | ||
| Sporoschisma mirabile* | FMR 11247 | Dead wood | Spain, Galicia, Enciña do Lastra P. | Sordariomycetes | HF937358 | HF677183 | HF677174 | |
| Stachybotrys cylindrospora | FMR 11595 | Dead wood | Spain, Castilla y León, Olleros del Alba | Sordariomycetes | KY853528 | KY853466 | ||
| Sterigmatobotrys uniseptata* | FMR 11937 | Dead wood | Spain, Aragón, Ordesa y Monte Perdido N.P. | Sordariomycetes | HF677182 | HF677178 | ||
| Sympodiella acicola | CBS 425.76 | Soil | Canada, Ontario, Muskoka | Dothideomycetes | KY853529 | KY853467 | ||
| CBS 487.82 | Needle of Pinus sylvestris | The Netherlands, Baarn, De Vuursche | Dothideomycetes | KY853530 | KY853468 | |||
| Trichocladium asperum | FMR 12054 | Soil | Spain, Castilla La Mancha, Alto Tajo P. | Sordariomycetes | KY853531 | KY853469 | ||
| CBS 903.85 | Soil | Germany, Edersee, Nieder-Werbe | Sordariomycetes | KY853532 | KY853470 | |||
| CBS 140.21 | Unknown | The Netherlands | Sordariomycetes | KY853533 | KY853471 | |||
| CBS 415.52 | Culture contaminant | UK, Cumberland | Sordariomycetes | KY853534 | ||||
| CBS 157.22 | unknown | unknown | Sordariomycetes | KY853535 | KY853472 | |||
| CBS 112.67 | Soil | Belgium, Kontich | Sordariomycetes | KY853536 | KY853473 | |||
| Triposporium deviatum* | FMR 13135, CBS 137300 | Dead wood | Spain, Canary Island, La Palma | Leotiomycetes | KY853537 | KY853474 | ||
| Troposporella fumosa | FMR 12437 | Dead wood | Spain, Cataluña, Alto Pirineo | Dothideomycetes | HF678544 | HF678534 | ||
| Vargamyces aquaticus | Xylomyces aquaticus | FMR 11587, CBS 130366 | Submerged wood | Spain, Burgos, Salas de Los Infantes | Dothideomycetes | KY853538 | KY853475 | |
| CBS 636.91 | Submerged wood | Hungary, Börzsöny Mts., Morgó stream | Dothideomycetes | KY853539 | ||||
| Vermiculariopsiella immersa | CBS 140223 | Rotten leaf | Spain, Canary Islands | Sordariomycetes | KY853540 | KY853476 | ||
| V.microsperma | CBS 101172 | Leaf litter | Brazil, Mata Atlantica, Engenho do Rei, Santa Rita | Sordariomycetes | KY853541 | KY853477 | ||
| CBS 140231 | Dead leaf | French Guiana | Sordariomycetes | KY853542 | KY853478 | |||
| V.pediculata* | FMR 12187, CBS 132484 | Twig | Portugal, Viana do Castello, Lagoas de Bertiandos, P.A. | Sordariomycetes | HF678537 | HF678527 | ||
| Xenospadicoides atra gen. et comb. nov. | Spadicoides atra | CBS 489.77 | Branch of Quercus petraea | Czech Republic, Central Bohemia, forest Lánská obora | Sordariomycetes | EF204521 | EF204506 | |
| Xyladictyochaeta lusitanica gen. et sp. nov.* | Dictyochaeta aff. eucalypti | FMR 12177, CBS 142290 | Dead leaves of Eucalyptus sp. | Portugal, Viana do Castello, Lagoas de Bertiandos, P.A. | Sordariomycetes | HF937349 | KY853543 | KY853479 |
| Zanclospora iberica sp. nov.* | Zanclospora aff. novae-zelandiae | FMR 11584, CBS 130426 | Dead wood | Spain, Asturias, Picos de Europa N.P. | Sordariomycetes | HF937360 | KY853544 | KY853480 |
| FMR 12186 | Bark of Eucalyptus sp. | Portugal, Viana do Castello, Lagoas de Bertiandos, P.A. | Sordariomycetes | HF937361 | KY853545 | KY853481 | ||
| Zygosporium gibbum* | FMR 13130, CBS 137306 | Dead leaves | Spain, Canary Island, La Palma | Sordariomycetes | KY853546 | KY853482 | ||
*Species without sequences previous to this study, †Species recently described from the Iberian Peninsula, bold: new species.
CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; FMR: Facultat de Medicina i Ciències de la Salut, Reus, Spain.
N.P.: National Park, P.: Natural Park, P.A.: Protected area.
SSU: partial small subunit of the rDNA; LSU: partial large subunit of the rDNA; ITS: internal transcribed spacer regions of the rDNA and 5.8S region.
Morphology
Morphological features were obtained from fungi growing on the natural substratum and on potato carrot agar (PCA; potatoes 20 g; carrots 20 g; agar 20 g; distilled water 1 L) or on oatmeal agar (OA; filtered oat flakes, 20 g agar, distilled water 1 L), incubated at 25 °C in the dark. Colony colours were assessed according to the charts of Rayner (1970). Measurements and descriptions of microscopic structures were taken from specimens mounted in either lactic acid or polyvinyl alcohol, using an Olympus BH-2 light microscope (Olympus Corporation, Tokyo, Japan). Lactophenol cotton blue was used as contrast colourant to examine hyaline structures. Photomicrographs were taken using differential interference contrast and phase contrast optics with a Zeiss Axio ImagerM1 light microscope (Zeiss, Oberkochen, Germany) and a DeltaPix Infinity X digital camera or a Nikon Eclipse Ni microscope, using a Nikon DS-U3 digital camera (Nikon, Tokyo, Japan) and NIS-Element imagining software v. 4.20.
DNA isolation, sequencing and phylogeny
Genomic DNA was extracted from fungal colonies using the FastDNA kit (MP Biomedicals, CA, USA) and PrepMan Ultra sample preparation reagent (Applied Biosystems, Foster City, CA, USA), following the manufacturer's protocols. Colonies were growing on OA or PCA at 25 °C for 1–2 wk; potato-dextrose broth (PDB) was used for those strains with slow growth. The primer sets NL1-NL4b (O'Donnell 1993), LROR-LR5 and ITS4-ITS5 (White et al. 1990) were used to amplify part of the nuclear rDNA operon spanning the 3′ end of the 18S rRNA gene, the first internal transcribed spacer (ITS1), the 5.8S rRNA gene, the second ITS region (ITS2) and ±700 bp of the 5′ end of the 28S rRNA gene (LSU), respectively. The small subunit of the rRNA (SSU) was amplified with the primers NS1–NS4 (White et al. 1990). The amplification cycles were performed following Cano et al. (2004). PCR products were purified and sequenced at Macrogen Corp. Europe (Amsterdam Zuid-Oost, the Netherlands) with an ABI PRISM 3730XL DNA Analyzer (Applied Biosystems, CA, USA). The program SeqMan v. 7.0 (Lasergene, Madison, WI, USA) was used to obtain consensus sequences.
The evolutionary relationships of the fungal isolates with other Ascomycota were mainly based on the analysis of the LSU and SSU gene sequences. Additional homologous sequences were retrieved from GenBank after a BLAST search and added in the phylogenetic analysis. Alignments were made using the web interface MAFFT v. 7 (Katoh & Standley 2013), followed by manual adjustments with MEGA v. 6 (Tamura et al. 2013) and a text editor. These alignments were deposited in TreeBASE (www.treebase.org) under the submission number S20197.
Phylogenetic reconstructions were performed using Maximum-Likelihood (ML) and Bayesian Inference (BI) approaches under RAxML HPC BlackBox v. 8.2.8 (Stamatakis 2014) using the Cipres Science gateway portal (Miller et al. 2010) and MrBayes v. 3.2.6 (Ronquist et al. 2012), respectively. Confident branch support is defined as Bayesian posterior probabilities (BPP) ≥ 0.95 and maximum likelihood bootstrap values (BML) ≥ 70 %.
Results and discussion
Phylogenetic relationships
Sequences (LSU, SSU and ITS) were determined for the isolates selected (Table 1), while their distribution in different orders and families in Pezizomycotina (Ascomycota) were highlighted by using LSU and SSU analyses (SSU tree not shown, available in TreeBASE). The taxa tested corresponded to four classes, i.e. Dothideomycetes, Eurotiomycetes, Leotiomycetes and Sordariomycetes. To maximise the quality of the alignment, four separate LSU alignments were created corresponding to the different fungal classes, although an additional dataset that includes the Savoryellales was used to resolve the phylogenetic relationship among the members of that order and allies. The number of taxa, characters and information generated from the BI and ML for each dataset are in Table 2. The consensus trees inferred from BI confirmed the tree topologies obtained from the ML analysis in all four datasets; therefore, only the BI consensus trees are shown (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5). Well-supported main clades are indicated in Arabic numbers, and the most relevant clades in each class discussed. Names in the phylogenetic trees are written after the taxonomic novelties introduced in the taxonomy section and old names are included in Table 1.
Table 2.
Number of taxa, characters and information generated from the Bayesian Inference and Maximum Likelihood analysis for each dataset.
| Dothideomycetes | Eurotiomycetes | Leotiomycetes | Sordariomycetes | Savoryellales and allies | |
|---|---|---|---|---|---|
| Number of taxa | 99 | 22 | 33 | 158 | 58 |
| Number of characters | 680 | 660 | 658 | 700 | 901 |
| Bayesian Inference | |||||
| Unique sites patterns | 469 | 280 | 279 | 544 | 550 |
| Substitution model used | GTR G+I | GTR G+I | GTR G+I | GTR G+I | GTR G+I |
| Maximum Likelihood analysis | |||||
| Constant characters | 284 | 403 | 421 | 234 | 426 |
| Parsimony informative sites | 307 | 191 | 228 | 358 | 370 |
| Variable and parsimony uninformative | 382 | 249 | 149 | 447 | 461 |
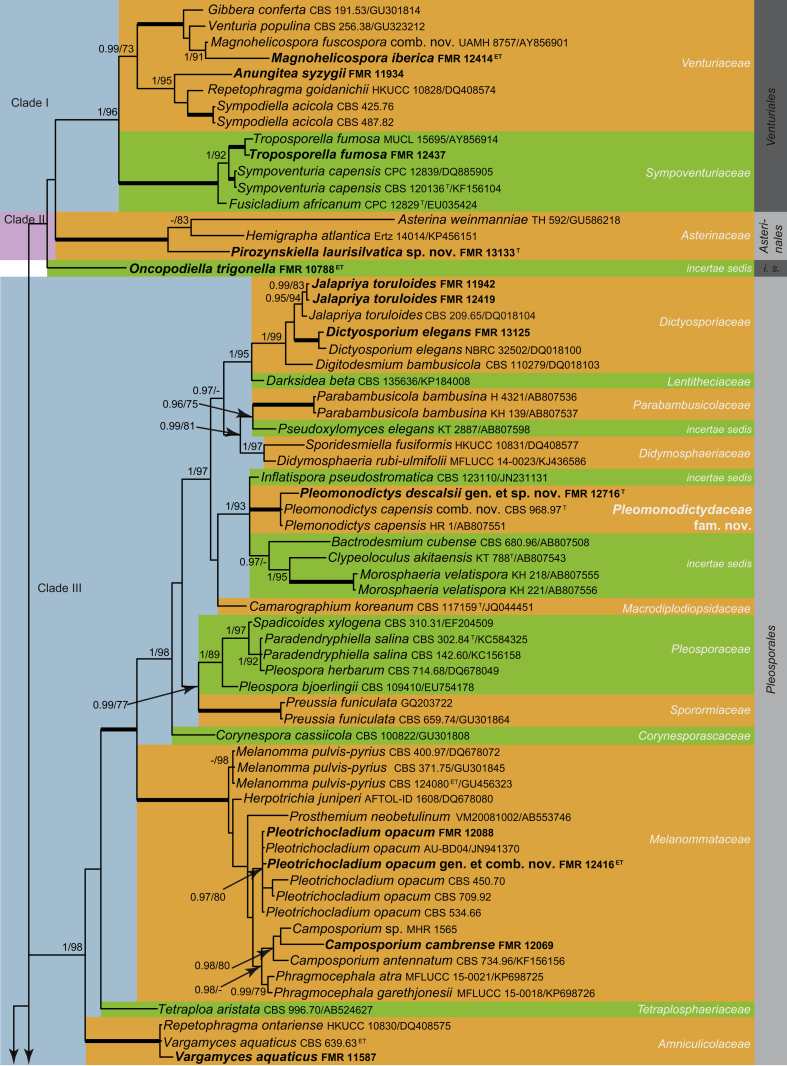
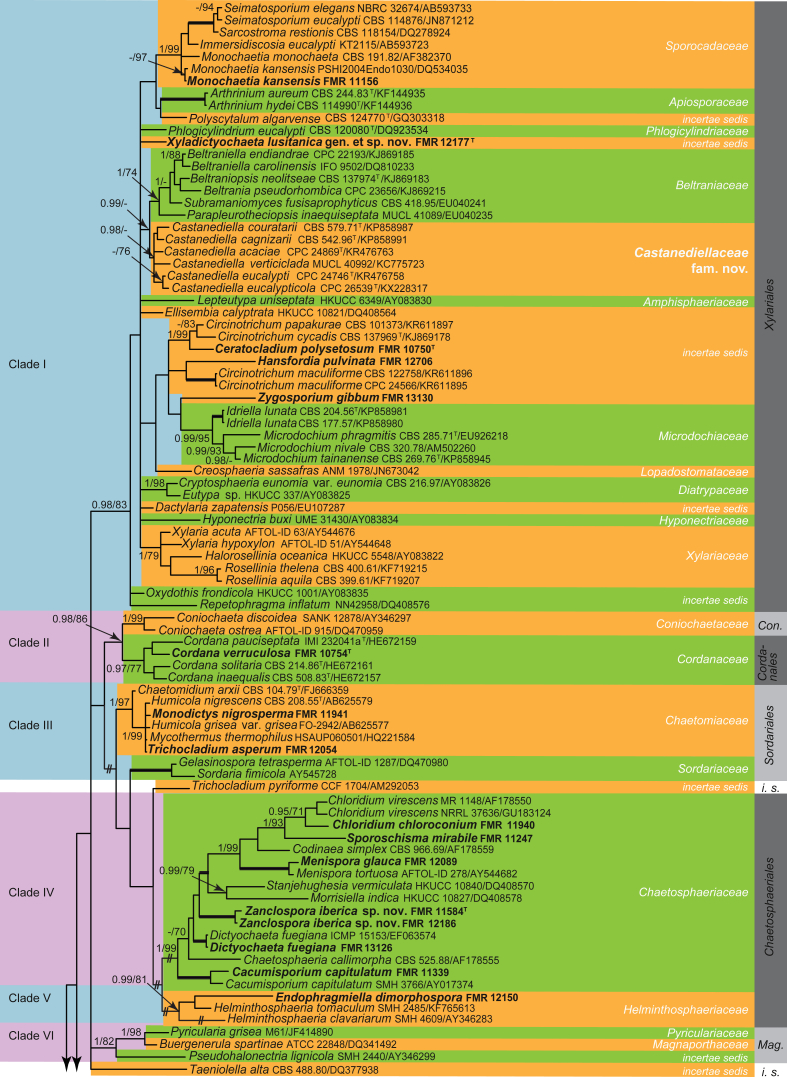
Fig. 1.
Phylogenetic tree inferred from the Bayesian analysis of the LSU sequences alignment of Dothideomycetes. Posterior probabilities inferred from the Bayesian analysis (≥0.95) and bootstrap (≥70 %) are indicated at the nodes of the branches (PP/BML). Thickened lines indicate a PP of 1.0 and a BML of 100 %. // indicates the branch was reduced 75 %. New taxa and species recently described from the Iberian Peninsula are indicated in bold. i. s. = incertae sedis; Wies. = Wiesneriomycetales. Tree was rooted with Saccharomyces cerevisiaeAH005487, Orbilia auricolorDQ470953 and O. vinosaDQ470952.
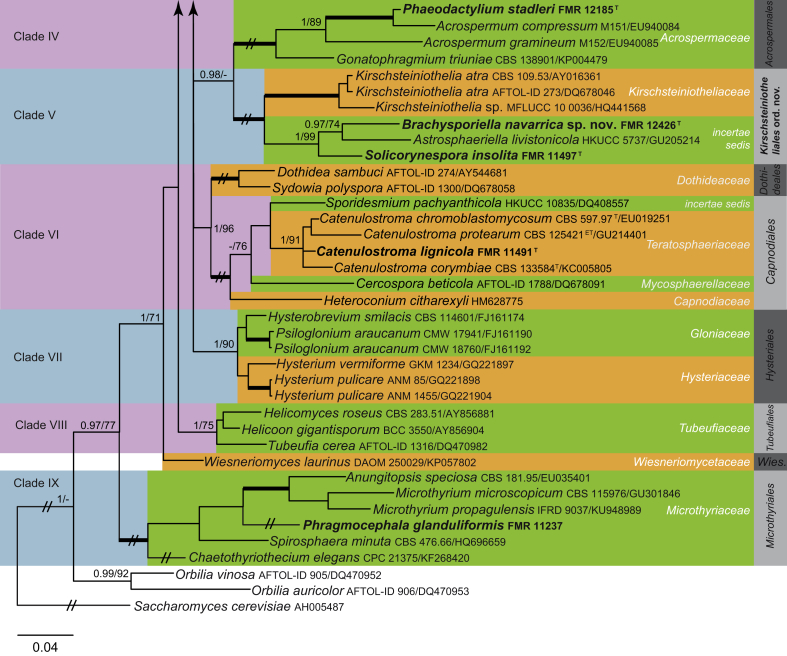
Fig. 2.
Phylogenetic tree inferred from the Bayesian analysis of the LSU sequences alignment of Eurotiomycetes. Posterior probabilities inferred from the Bayesian analysis (≥0.95) and bootstrap (≥70 %) are indicated at the nodes of the branches (PP/BML). Thickened lines indicate a PP of 1.0 and a BML of 100 %. // indicates the branch was reduced 75 %. New taxa and species recently described from the Iberian Peninsula are indicated in bold. Tree was rooted with Saccharomyces cerevisiaeAH005487, Orbilia auricolorDQ470953 and O. vinosaDQ470952.
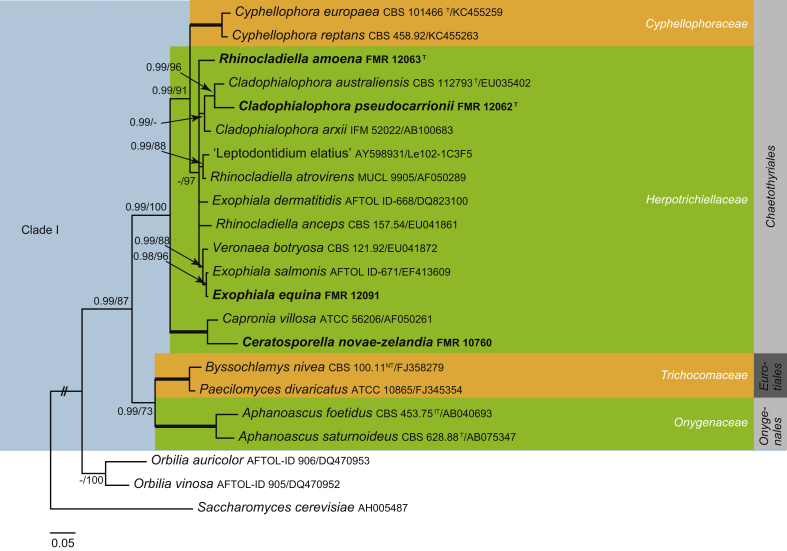
Fig. 3.
Phylogenetic tree inferred from the Bayesian analysis of the LSU sequences alignment of Leotiomycetes. Posterior probabilities inferred from the Bayesian analysis (≥0.95) and bootstrap (≥70 %) are indicated at the nodes of the branches (PP/BML). Thickened lines indicate a PP of 1.0 and a BML of 100 %. // indicates the branch was reduced 75 %. New taxa and species recently described from the Iberian Peninsula are indicated in bold. Tree was rooted with Saccharomyces cerevisiaeAH005487, Orbilia auricolorDQ470953 and O. vinosaDQ470952.
Fig. 4.
Phylogenetic tree inferred from the Bayesian analysis of the LSU sequences alignment of Sordariomycetes. Posterior probabilities inferred from the Bayesian analysis (≥0.95) and bootstrap (≥70 %) are indicated at the nodes of the branches (PP/BML). Thickened lines indicate a PP of 1.0 and a BML of 100 %. // indicates the branch was reduced 75 %. New taxa and species recently described from the Iberian Peninsula are indicated in bold. Con. = Coniochaetales, i. s. = incertae sedis, Mag. = Magnaporthales, Trich. = Trichosphaeriales, Glom. = Glomerellales, Micr. = Microascales. Tree was rooted with Saccharomyces cerevisiaeAH005487, Orbilia auricolorDQ470953 and O. vinosaDQ470952.
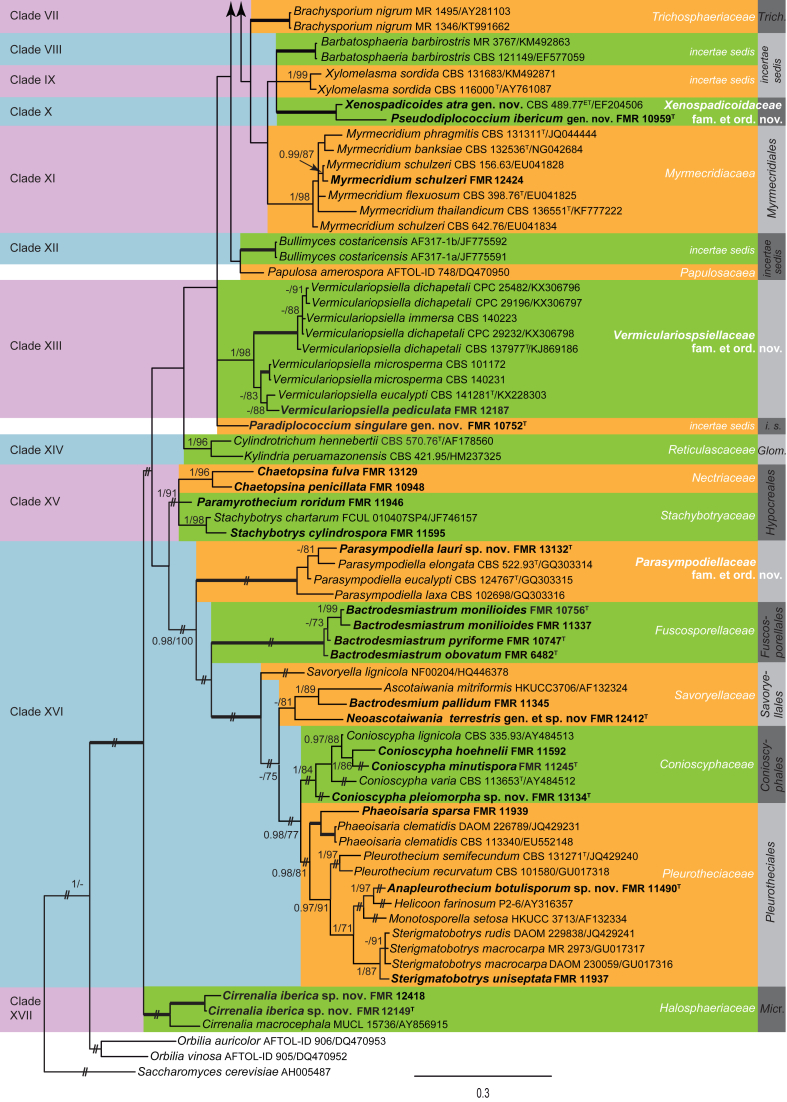
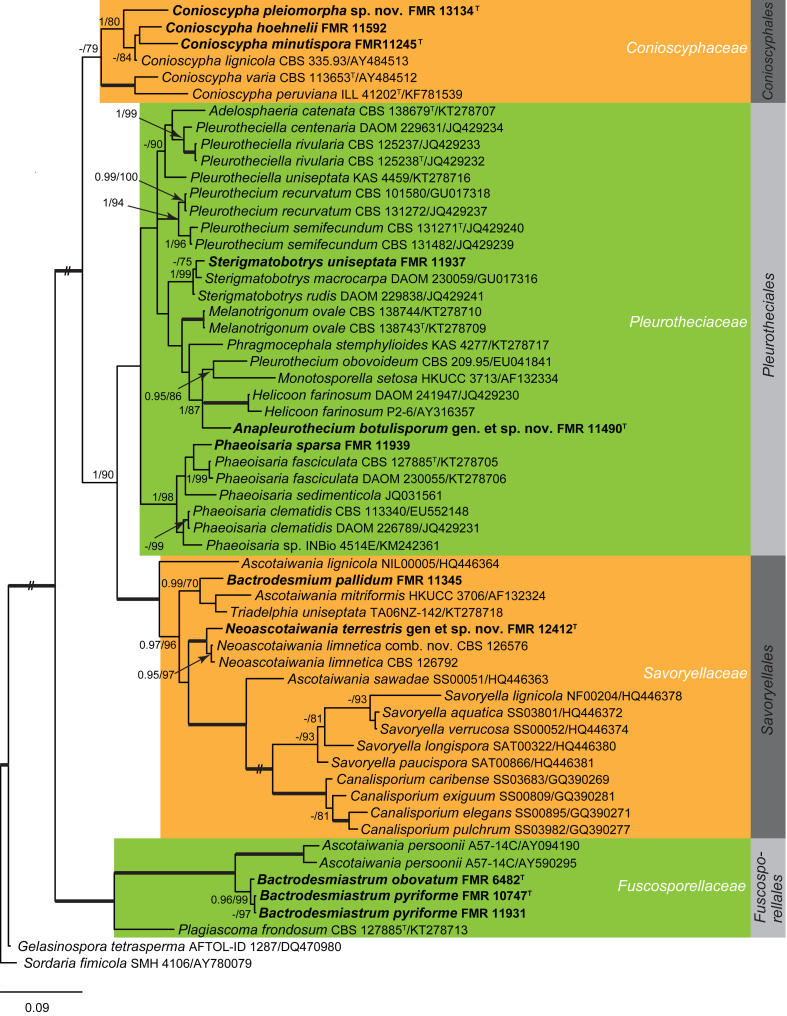
Fig. 5.
Phylogenetic tree inferred from the Bayesian analysis of the LSU sequences alignment of Savoryellales and allies. Posterior probabilities inferred from the Bayesian analysis (≥0.95) and bootstrap (≥70 %) are indicated at the nodes of the branches (PP/BML). Thickened lines indicate a PP of 1.0 and a BML of 100 %. // indicates the branch was reduced 75 %. New taxa and species recently described from the Iberian Peninsula are indicated in bold. Tree was rooted with Gelasinospora tetrasperma (DQ470980) and Sordaria fimicola (AY780079).
Dothideomycetes
Within Dothideomycetes, a total of nine strongly supported main clades (I–IX) were discerned, which showed a moderately supported backbone (0.97 PP, 77 % BS).
Clade I represents the Venturiales (1 PP, 96 % BS), in which Magnohelicospora iberica (FMR 12414), Anungitea syzygii (FMR 11934) and Troposporella fumosa (FMR 12437) are included. Magnohelicospora iberica was placed in a subclade together with Venturia populina and Gibbera conferta (1 PP, 100 % BS). Magnohelicospora is a monotypic genus characterised by polyblastic conidiogenous cells producing single brown helicoidal conidia with a conidial filament coiled in three dimensions (Castañeda-Ruiz et al. 2012). It is morphologically similar to Helicodendron (Hd.) and Helicoon (Ho.). However, the conidia in Helicodendron are catenate, while in Helicoon they are produced singly on monoblastic conidiogenous cells. It is noteworthy that Helicodendron and Helicoon are polyphyletic (Tsui & Berbee 2006). For instance, Hd. paradoxum, the type species of the genus, and Hd. giganteum are both placed in Helotiales (Leotiomycetes) (Tsui & Berbee 2006) in which they are linked to sexual morphs of different genera, i.e. Hymenoscyphus and Mollisia, respectively (Fisher & Webster 1983). As for M. iberica, Hd. pinicola, the asexual morph of Tyrannosorus pinicola (Untereiner et al. 1995), is also related to Venturiaceae, specifically to Caproventuria (Zhang et al. 2011). Furthermore, M. iberica and Hd. pinicola differ in the arrangement of conidia, solitary in the former and catenate in the latter.
Helicoon species are scattered in three classes of Ascomycota, i.e. Leotiomycetes, Dothideomycetes and Sordariomycetes. Based on cultural methods, Ho. sessile, the generic type, was associated to the sexual morph Orbilia luterubella (Orbiliaceae, Leotiomycetes) (Pfister 1997). Helicoon farinosum was shown to be the asexual morph of an ascotaiwania-like fungus (Fallah et al. 1999) and belongs to Pleurotheciales (Sordariomycetes) (Réblová et al. 2016b) (Fig. 4, clade XVI, Fig. 5). Based on the analysis of LSU and ITS sequences, Tsui & Berbee (2006) placed Ho. gigantasporum in Tubeufiaceae, Ho. richonis in Pleosporales and Ho. fuscosporum as incertae sedis in Dothideomycetes. Our analysis suggests that Ho. fuscosporum, placed in Venturiaceae (Fig. 1, clade I), is congeneric with M. iberica and a new combination is proposed. However, the redisposition of other species in Helicodendron and Helicoon and their related sexual morphs require further re-evaluation.
Anungitea syzygii (FMR 11943) is placed in a subclade together with Repetophragma goidanichii (DQ408574) and Sympodiella acicola (CBS 425.76 and CBS 487.82). Other LSU sequences which were similar to that of A. syzygii were Cylindrosympodium lauri (EU035414, 96 %) and Tothia fuscella (JF927786, 95 %). Anungitea was established by Sutton (1973) with A. fragilis as type species. It is characterised by straight, simple and brown conidiophores with polyblastic, sympodial, denticulate conidiogenous cells and cylindrical, hyaline conidia produced in acropetal chains (Seifert et al. 2011). Cylindrosympodium lauri is morphologically similar to A. syzygii in having solitary conidiophores with polyblastic, sympodial conidiogenous cells. Nevertheless, they are different in conidial morphology; C. lauri produces solitary, subacicular to narrowly subcylindrical, (4–)6–8-septate conidia (Crous et al. 2007b), while A. syzygii produces short chains of cylindrical, 0–1-septate conidia. On the other hand, R. goidanichii shows conidiophores with terminal and monoblastic conidiogenous cells extending percurrently and producing solitary and brown phragmoconidia. Thotia fuscella has thyriothecial ascomata with no asexual morph reported (Wu et al. 2011). The placement of Anungitea within the Venturiaceae was previously suggested by Crous et al. (2007a) by comparing morphological similarities with the genus Fusicladium. However, A. fragilis, the type species of Anungitea, has no preserved ex-type strain to confirm the definitive position of the genus.
Another helicosporous genus placed in the clade I (Fig. 1) is Troposporella. Troposporella, typified by T. fumosa (Karsten 1892), is characterised by producing brown sporodochial conidiomata, undifferentiated conidiophores, polyblastic, pale brown conidiogenous cells and helicoidal conidia. Tsui & Berbee (2010) showed that T. fumosa, T monilipes and T. olivaceum formed a monophyletic Troposporella clade incertae sedis in Dothideomycetes. The LSU and ITS sequences of our isolate of T. fumosa are respectively 99 % (524/527) and 98 % (525/535) similar with sequences of other conspecific specimens (accession numbers AY856914 and DQ351724, respectively) included in the mentioned study. According to our results, the genus Troposporella belongs to the Sympoventuriaceae, Venturiales (1 PP, 100 % BS).
Clade II includes Asterina weinmanniae and Hemigrapha atlantica, members of Asterinales, and the isolate FMR 13133, which shows, however, morphological and ecological affinities with Pirozynskiella. Pirozynskiella, typified by P. solaninum, is only known by an asexual morph characterised by brown conidiophores with blastic conidiogenous cells bearing a single unbranched acropetal chain of ellipsoidal to subcylindrical conidia (Hughes 2007). Our isolate FMR 13133 differs from other species of Pirozynskiella mainly by the verrucose conidia and, therefore, it is described below as P. laurisilvatica sp. nov. Because no phylogenetic analyses have been done that include the type of Pirozynskiella, the taxonomic placement of the genus remains uncertain.
Our LSU and SSU sequence data reveal that Oncopodiella trigonella (FMR 10788) is unrelated to any previously established order or family in Dothideomycetes, and represents a potentially new lineage in this class. In order to stabilise the use of this name we propose the strain FMR 10788 as the epitype of O. trigonella since the generic type has no preserved cultures.
Clade III comprises members of Pleosporales (1 PP, 98% BS). The Iberian isolates related to this clade include Camposporium cambrense (FMR 12069), Dictyosporium elegans (FMR 13125), Jalapriya toruloides (FMR 11942 and FMR 12491), Monodictys sp. (FMR 12716), Trichocladium opacum (FMR 12416 and FMR 12088), and Vargamyces aquaticus (FMR 11587). Dictyosporium elegans and Jalapriya toruloides are placed in the Dictyosporiaceae subclade (Fig. 1, 1 PP, 98 % BS), a pleosporalean family introduced recently by Boonmee et al. (2016) that includes fungi with cheiroid or digitate, palmate and/or dictyosporous conidia. Dictyosporium and Jalapriya are very similar, sharing sporodochial conidiomata composed of slightly differentiated conidiophores which produce brown complanate cheiroconidia. Nevertheless, based on phylogenetic differences they were proposed as different genera (Boonmee et al. 2016).
The isolate FMR 12176, identified as Monodictys sp. due to its dark brown and dictyosporous conidia, is included in a well-supported clade (1 PP, 100 % BS) with two isolates of Monodictys capensis, including the ex-type strain (CBS 968.97), a fungus considered incertae sedis at family level by Tanaka et al. (2015). Monodictys is characterised by single, brown muriform conidia originating from monoblastic cylindrical conidiogenous cells (Ellis 1971). However, these characters have limited taxonomic value because Monodictys species are scattered in different classes, i.e. Dothideomycetes, Sordariomycetes and Leotiomycetes (Tanaka et al. 2015). According to our analysis, for instance, M. nigrosperma (FMR 11941) is placed in the Chaetomiaceae (Sordariales) (Fig. 4). Although the phylogenetic placement of the type species of Monodictys, M. putredinis, remains unclear, this species was reported as the asexual morph of Ohleria brasilensis (Samuels 1980). Ohleria was recently included in the Ohleriaceae, Pleosporales (Jaklitsch & Voglmayr 2016), which is distant from the M. capensis clade. Our results agree with the phylogeny presented by Tanaka et al. (2015), and support the proposal of the new genus Pleomonodictys for these two monodictys-like species. This lineage also represents a new family in the Pleosporales, Pleomonodictydaceae fam. nov.
Our isolates of Trichocladium opacum and several strains of the same species clustered in a supported clade (0.97 PP, 80 % BS) of the family Melanommataceae (Fig. 1, clade III). This fungus is characterised by conidiophores reduced to conidiogenous cells, producing ellipsoidal to clavate, dark brown conidia with schizolytic secession. Since the type species of the genus Trichocladium, T. asperum, is related to the Chaetomiaceae in the Sordariomycetes (Fig. 4, clade III), T. opacum clearly represents a different and distinct pleosporalean genus which is introduced below as Pleotrichocladium.
Camposporium cambrense forms a subclade (0.98 PP, 80 % BS) together with C. antennatum (CBS 734.96, GenBank KF156156 as Paradendryphiella salina) and Camposporium sp. (MHR 1565) in the Melanommataceae. Camposporium was erected by Harkness (1884) and typified with C. antennatum. It is characterised by dematiaceous, unbranched conidiophores with terminal, integrated, denticulate conidiogenous cells that produce multiseptate cylindrical conidia, either with or without hyaline appendages at the apex and a persistent portion of the denticle attached at the base. Our analysis shows for the first time that Camposporium is related to the Pleosporales.
Vargamyces aquaticus (FMR 11587 and CBS 639.63) and Repetophragma ontariense (GenBank DQ408575) also formed a full-supported subclade in Pleosporales. Repetophragma ontariense was previously shown to be related to the Amniculicolaceae, which includes saprobic freshwater fungi (Zhang et al., 2009a, Zhang et al., 2009b). FMR 11587 also was isolated from a freshwater habitat. Recently, Révay et al. (2014) suggested that V. aquaticus and R. ontariense could be considered conspecific, but they did not introduce any taxonomic change. Based on morphological and genetic similarity, we regarded R. ontariense as synonym of V. aquaticus.
The fully supported clade IV includes species of Acrospermum, Gonatophragmium triuniae and Phaeodactylium stadleri, the latter being sequenced for the first time in the present study. According to our analysis, this clade represents the Acrospermales (Minter & Watson 2007). Acrospermum species are saprophytic fungi found on different substrates, with an asexual morph characterised by unbranched, pale brown conidiophores, with polyblastic, scattered conidiogenous cells that produce hyaline to pale brown, smooth conidia (Webster, 1956, Minter and Watson, 2007). Phaeodactylium stadleri resembles the asexual morph of Acrospermum, but differs in having branched conidiophores and verrucose conidia (Castañeda-Ruiz et al. 2012). Phaeodactylium is also similar to Gonatophragmium having branched conidiophores with polyblastic denticulate conidiogenous cells. However, in Gonatophragmium conidiophores are unilateral nodose and darker than those observed in Phaeodactylium. Unfortunately, there are no DNA sequence data of the type species of Phaeodactylium, P. venkatesanum, making it impossible to resolve its phylogeny.
Clade V (1 PP, 100 % BS) includes members of the Kirschsteiniotheliaceae and other fungi of uncertain taxonomic position, i.e. Solicorynespora insolita, Astrosphaeriella livistonicola and an isolate of Brachysporiella sp. (FMR 12426). With the exception of A. livistonicola, for which no asexual morph is known (Hyde & Fröhlich 1997), the asexual morphs of most members of this clade (i.e. Brachysporiella, Kirschsteiniothelia (=Dendryphiopsis), Solicorynespora s.l., Sporidesmium s.l. and Taeniolella s.s.) are characterised by pigmented conidiogenous cells and septate, dark brown conidia (Shearer et al., 2009, Hernández-Restrepo et al., 2014a, Ertz et al., 2016). Brachysporiella has macronematous conidiophores, mono- and polyblastic conidiogenous cells and dark brown, septate and terminal conidia (Batista, 1952, Ellis, 1971). The isolate FMR 12426 shares these features, but differs in some aspect from the other species accepted in Brachysporiella. It is therefore described as B. navarrica sp. nov. Astrosphaeriella is polyphyletic, with species scattered in different pleosporalean lineages (Liu et al., 2011, Zhang et al., 2012). Since clade V is distantly related to other lineages representative of different orders in Dothideomycetes, it is introduced below as the new order Kirschsteiniotheliales.
Clade VI includes members of Capnodiales and Dothideales (Fig. 1, 1 PP, 96 % BS). The isolate FMR 11491 belongs to the former. This isolate was morphologically regarded as a putative species of Heteroconium. However, in the analysis it appears closely related to three species of Catenulostroma (1 PP, 91 % BS), i.e. C. corymbiae, C. chromoblastomycosum and to C. protearum, the generic type. Catenulostroma and Heteroconium are morphologically similar in having dark brown conidiophores, with catenate, brown phragmoconidia. Nevertheless, species of Catenulostroma have hypha-like conidiophores and conidia in basipetal chains (Crous et al. 2007a), whereas in Heteroconium conidiophores are differentiated, bearing an apical metula, and conidia are born in acropetal chains (Hughes 2007). Catenulostroma belongs to the Teratosphaeriaceae (Crous et al., 2007a, Quaedvlieg et al., 2014), while Heteroconium is polyphyletic (Cheewangkoon et al. 2012). Heteroconium s.s. (based on the type species, H. citharexyli) belongs to the Capnodiaceae but other species are of uncertain affinities within Dothideomycetes or allocated to new genera, as Alysidiella eucalypti and A. kleinziense, both occurring on Eucalyptus (Cheewangkoon et al. 2012). Due to the phylogenetic affinity of FMR 11491 with Catenulostroma and considering its morphological differences from the currently accepted species, C. lignicola sp. nov. is introduced.
Clade IX (1 PP, 100 % BS) groups Phragmocephala glanduliformis (FMR 11237) with members of the Microthyriaceae (Microthyriales). The former is placed in a long single branch distantly related to the lineages representing Mycrothyrium and Anungitopsis speciosa. Phragmocephala (Ph.) was erected by Mason & Hughes (1951), with Ph. cookei as type species. It is characterised by dark brown conidiophores and holoblastic, dark brown and septate conidia. Currently, Phragmocephala includes nine species (Seifert et al. 2011) and, in addition to Ph. glanduliformis (Microthyriaceae), phylogenetic data are only known from Ph. atra, Ph. garethjonesii (Melanommataceae) (Su et al. 2015), and Ph. stemphylioides (Pleurotheciaceae) (Réblová et al. 2016b). Hence Phragmocephala is another polyphyletic genus that needs further study.
Eurotiomycetes
Four Iberian isolates were related to the Herpotrichiellaceae (Fig. 2), i.e. Ceratosporella novae-zealandiae (FMR 10760), Cladophialophora pseudocarrionii (FMR 12062), Exophiala equina (FMR 12091) and Rhinocladiella amoena (FMR 12063). Members of this family include numerous black-yeast fungi of clinical relevance such as Exophiala, Cladophialophora, Phialophora, and Rhinocladiella (de Hoog et al. 2011), but also fungi isolated from soil, plant debris and water (Réblová et al. 2013). With the exception of C. novae-zealandiae, the other fungi have been previously studied phylogenetically. Exophiala equina belongs to the Exophiala salmonis-clade. It has been reported as etiologic agent of subcutaneous infections in animals (horses and turtles) and humans, but it has been also isolated from water, soil and plant material (de Hoog et al. 2011). Cladophialophora pseudocarrionii and Rhinocladiella amoena have been recently described by Madrid et al. (2016) from soil and submerged wood, respectively. Ceratosporella novae-zealandiae formed a distinct linage, related to Capronia villosa (GenBank AF050261 sequences from ex-type strain) (1 PP, 100 % BS). Capronia villosa is characterised by setose perithecial ascomata and an exophiala-like asexual morph (Müller et al. 1987), features that clearly distinguish this fungus from Ceratosporella. Ceratosporella includes species with macronematous unbranched conidiophores, with monoblastic, percurrent conidiogenous cells, which produce solitary, branched, and brown to dark brown conidia. The generic type, C. bicornis, is a parasitic fungus on Zea mays, although most species in the genus are saprophytic (Hughes, 1971, Matsushima, 1993, Castañeda-Ruiz et al., 1996). Our result represents a novel phylogenetic scenario in which asexual fungi with branched conidia are reported within this order, which in addition could represent an undescribed family in the Chaetothyriales. However, no DNA sequence is available, neither for the type species of Ceratosporella nor for any of the 90 species included in the genus. Therefore, the phylogenetic placement of the genus remains uncertain until new molecular data for these fungi can be provided.
Leotiomycetes
The Iberian isolates related to the Leotiomycetes (Fig. 3) were distributed in different clades among members of the Helotiales (Clade I–VIII), except the clade IX which could represent a different order. The order Helotiales lacks sufficient genetic data and the placement of several genera at family level is, in many cases, based on morphological criteria (Jaklitsch et al. 2016a).
Clade I (1 PP, 100 % BS) is composed of Diplococcium spicatum (EF204497), Trimmatostroma salicis (EU019300) and an unidentified isolate FMR 11236. Previous phylogenetic analyses have shown that Diplococcium and Trimmatostroma belong to the Helotiales and more specifically to the Mollisiaceae (Crous et al., 2015b, Jaklitsch et al., 2016a). These two genera are characterised by producing catenate conidia, while the Iberian isolate produces solitary conidia. In addition, D. spicatum exhibits differentiated conidiophores, polytretic conidiogenous cells and 1-septate conidia (Ellis 1971), whereas T. salicis has meristematic, arthric conidiogenous cells, producing chains of phragmo- or dictyoconidia (Seifert et al. 2011). FMR 11236 has meristematic, blastic conidiogenous cells, and solitary, black to dark brown conidia formed by masses of rounded to angular cells. Therefore, these peculiar morphological features and its phylogenetic distance with the fungi compared support the introduction below of Fuscosclera gen. nov.
In our phylogenetic tree (Fig. 3), Bloxamia truncata, the type species of the genus, was placed on an isolated branch, which we recognise as representative of the previously proposed family Bloxamiaceae (Locquin 1984). Since this family name was invalidly published (Art. 39.1; no Latin diagnosis), we validate Bloxamiaceae in the taxonomy section. Jaklitsch et al. (2016a) treated Bloxamiaceae as a presumed synonym of Pezizellaceae. However, in our phylogenetic analysis, Pezizellaceae (Fig. 3, Clade VII), which contains members of Chalara, is distantly related to the Bloxamia lineage. Bloxamia truncata is characterised by sporodochial conidiomata, phialidic conidiogenous cells and hyaline, rectangular conidia held in readily disarticulating chains. It has been associated with the sexual morph Bisporella sulfurina (Johnston 1988), but we have not been able to confirm this based on our isolate. The genus Bisporella (Bis.) was established by Korf & Carpenter (1974) with Bis. pallescens as generic type. Unfortunately, cultures and sequences of this species are not available. Additional studies are needed to clarify the phylogenetic relationship of those genera.
In our analyses the Hyaloscyphaceae appears polyphyletic, with members distributed in two well-supported, distant clades (II and IV). The clade II includes Hyaloscypha aureliella (AB546943) and sequences of two Iberian isolates that are cheiromycella-like (FMR 11559 and FMR 10851). Hyaloscypha sexual morphs are characterised by minute, sessile, white apothecia; while the asexual morphs have holoblastic or enteroblastic conidiogenesis that are cheiromycella-, pseudaegerita- and phialophora-like (Huhtinen, 1989, Quijada et al., 2017). Hyaloscypha aureliella asexual morph (= Cheiromycella microscopica) is characterised by brown sporodochia and simple, but usually branched, cheiroid, brown conidia (Ellis 1971). These conidia have usually been reported with two to three rows of cells (Ellis, 1971, Sutton, 1985); however, our isolates show conidia predominantly with one row of cells. Despite the morphological differences observed, considering the high similarity of their ITS and LSU sequences (99 % similarity with both markers) we identified both isolates as H. aureliella.
Clade IV (1 PP, 100 % BS) includes isolate FMR 11585 identified as Haplographium delicatum, and the sequence of Haplographium catenatum CBS 482.67 (FJ839656). Haplographium delicatum is the asexual morph of Dematioscypha dematiicola (Huhtinen 1987). Since the latter species is the type of Dematioscypha and this genus is preserved against Haplographium, H. delicatum is currently named D. dematiicola (Johnston et al. 2014). On the other hand, the taxonomy of H. catenatum seems to be controversial. Although initially included in Haplographium (Holubová-Jechová 1973), it was transferred to the genus Lauriomyces because of the production of conidia in chains (Castañeda-Ruiz & Kendrick 1990). Our phylogeny agrees in placing this species in Dematioscypha rather than Lauriomyces (Fig. 3, clade IX). However, since the identity of CBS 482.67 is unclear, an extensive study including more isolates of the species and allied taxa is necessary to confirm the taxonomy of this fungus.
Clade V comprises a fully supported lineage with two species of Triposporium (Tp.), Tp. cycadicola (KJ869177) previously reported in Helotiales by Crous et al. (2014b), and Tp. deviatum (FMR 13135). Species of this genus are characterised by straight or flexuous, brown conidiophores, monoblastic, integrated, terminal, percurrent, cylindrical, doliiform or lageniform conidiogenous cells and the conidia are branched, with 3–4 smooth, septate arms. Triposporium was erected by Corda (1837) with Tp. elegans as generic type and placed in the Triposporiaceae according to Nannizzi (1934). However, since Tp. elegans has never been sequenced, the placement of the genus in Helotiales is provisional.
Clade VII (1 PP, 98 % BS) includes Chalara hughesii (FMR 12413), Ch. kendrickii (AF222464) and Ch. aurea (AF222449). Chalara is a heterogeneous genus characterised by sessile or stalked, usually pigmented phialides with a basal venter and a long cylindrical collarette. The conidia are mostly hyaline, catenate, cylindrical, 1–2- or occasionally multi-celled (Nag-Raj & Kendrick 1975). The type species Ch. fusidioides was originally described as Torula fusidioides from bark of a conifer in Bohemia (Corda 1838); however, no holotype was designated in the protologue. Based on SSU and LSU sequence data, Cai et al. (2009) demonstrated that Chalara was polyphyletic within the Helotiales.
Clade VIII (1 PP, 76 % BS) is composed by Leptodontidium trabinellum, the type species of the genus (CBS 329.53 ex-type), L. irregulare (CBS 851.73 ex-type) and Leptodontidium sp. FMR 11834. Leptodontidium is characterised by grey to black funiculose colonies, hyaline conidiogenous cells, and small conidia formed more or less sympodially; it currently includes 10 species (De Hoog and Hermanides-Nijhof, 1977, Seifert et al., 2011). Morphological features of FMR 11834 do not fit with any of the species described in the genus and it is therefore introduced here as L. aureum sp. nov. This lineage is distantly related to other helotialean families and it is sufficiently distinct to be recognised as a new family, Leptodontidiaceae fam. nov.
Clade IX is represented by Lauriomyces (La.) bellulus and La. helicocephalus. Lauriomyces, typified by La. pulcher, is characterised by penicillate, brown conidiophores, sympodial denticulate conidiogenous cells, branched acropetal chains of hyaline conidia and longer basal ramoconidia, found commonly on dead leaves (Castañeda-Ruiz & Kendrick 1990). According to our tree (Fig. 3), Lauriomyces represents a fully supported independent lineage (1 PP, 100% BS) basal to the Helotiales, and here we introduce a new order and family, Lauriomycetales ord. nov., Lauriomycetaceae fam. nov. to accommodate it.
Sordariomycetes
A total of 52 % of the Iberian isolates belong to the Sordariomycetes, dispersed into 17 clades (Fig. 4, clades I–XVII). The backbone of this class was highly supported (1 PP, 100 % BS) with the LSU phylogeny.
Clade I is represented by members of Xylariales (0.98 PP, 83 % BS), including Ceratocladium polysetosum (FMR 10750), Hansfordia pulvinata (FMR 12076), Monochaetia kansensis (FMR 11156), Zygosporium gibbum (FMR 13130) and the unidentified isolate FMR 12177. With the exception of M. kansensis, the mentioned fungi are of uncertain position in the order.
Monochaetia kansensis is a pestalotioid fungus causing leaf spots in plants, characterised by dark acervular conidiomata and phragmoconidia with brown central cells and hyaline apical cells, bearing appendages. Traditionally, Monochaetia was treated as a member of Amphisphaeriaceae, but recently it has been placed in Sporocadaceae (Jaklitsch et al. 2016b).
The strain FMR 12177, collected from fallen leaves of Eucalyptus sp., is similar to Dictyochaeta eucalypti from which it differs, however, mainly in the presence of polyphialidic conidiogenous cells with inconspicuous collarettes. Dictyochaeta eucalypti has setiform conidiophores, with intercalary and terminal monophialidic conidiogenous cells with conspicuous collarettes, which are born directly on the conidiophore (Sutton & Hodges 1975). Although no sequence of this species is available to infer its affinities, several molecular studies include Dictyochaeta species in the Chaetosphaeriaceae (Réblová, 2004, Fernández et al., 2006). The isolated position of FMR 12177 among the Xylariales and its morphological peculiarities support the introduction of the new genus Xyladictyochaeta.
In the present analysis, several species of Castanediella, including the type, C. acaciae, form a monophyletic clade, sister to the Beltraniaceae lineage. Since it is a supported undescribed lineage in Xylariales, it is introduced here as Castanediellaceae fam. nov.
Ceratocladium (Ce.) polysetosum (Mena-Portales et al. 2011) is closely related to Circinotrichum (Ci.) papakurae and Ci. cycadis. These species form a well-supported clade (1 PP, 99 % BS), that is, however, distantly related to the Circinotrichum s.s. lineage, represented by Ci. maculiforme, the generic type. Unfortunately, there are no molecular data from other Ceratocladium species to infer their affinities and the mono- or polyphyletic nature of the genus as presently circumscribed. Ceratocladium and Circinotrichum are morphologically similar, they are both characterised by dark setae, polyblastic, lageniform conidiogenous cells and unicellular hyaline conidia. They differ in the branching pattern of the setae, simple in Circinotrichum and apically branched in Ceratocladium. The taxonomic value of the setae branching pattern needs to be assessed. In our analysis, the Circinotrichum s.s. lineage nested with Hansfordia pulvinata in an unsupported subclade. This latter species is characterised by macronematous and branched conidiophores with terminal polyblastic conidiogenous cells, producing globose to subglobose hyaline conidia. The lack of molecular data for most of the species of Ceratocladium, Circinotrichum or Hansfordia hinders the elucidation of taxonomic groups in these fungi.
Zygosporium gibbum formed a separate and independent lineage in Xylariales. Zygosporium is typified with Z. oscheoides and characterised by darkly pigmented, incurved vesicular cells usually born from the side of setiform conidiophores; the vesicles may be stalked or sessile, and give rise to 2–4 ampulliform conidiogenous cells that produce aseptate, ellipsoid or globose, smooth or variously ornamented conidia (Mason, 1941, Hughes, 1951). This is the first report of Zygosporium in Xylariales. However, the phylogeny of this genus remains uncertain pending further studies including more isolates and molecular markers.
Clade II (0.98 PP, 86 % BS) includes members of Coniochaetales and Cordanales distributed in two well- (1 PP, 99 % BS) and moderate-supported (0.97 PP, 77 % BS) subclades. Two species of Cordana were collected from plant debris during our sampling and described as C. mercadiana and C. verruculosa (Hernández-Restrepo et al. 2014b). Only C. verruculosa is included in the present phylogenetic analysis. Cordanales was recently introduced for species of Cordana (Hernández-Restrepo et al. 2015b).
Clade III is represented by members of the Sordariales. The Chaetomiaceae forms a strongly supported subclade (1 PP, 97 % BS), which includes several poorly discriminated genera using LSU data (Wang et al., 2016a, Wang et al., 2016b). Two Iberian isolates, Monodictys nigrosperma (FMR 11941) and Trichocladium asperum (FMR 12054), were included in Chaetomiaceae. Monodictys and Trichocladium are two heterogeneous and polyphyletic genera (Mantle et al. 2006, Tanaka et al. 2015) (also in Dothideomycetes, Fig. 1, clade III). Although, the two genera share conidiophores slightly differentiated, with holoblastic conidiogenous cells and dark brown conidia, they can be differentiated by conidial features; T. asperum has subglobose to oval or cylindrical-oval, transversely septate and coarsely warted conidia, whereas M. nigrosperma has smooth, clavate to pyriform conidia, with longitudinal and transverse septa. Trichocladium s.s. based on the type species, T. asperum, was previously shown to be related to the Sordariales close to Chaetomium and Humicola (Hambleton et al. 2005), although Mantle et al. (2006) considered T. asperum as member of Calosphaeriales. Our results agree with Hambleton et al. (2005) relating T. asperum with Sordariales, specifically with the Chaetomiaceae.
This is the first report of the phylogenetic affinity of M. nigrosperma with the Chaetomiaceae. Monodictys s.s., based on M. putredinis, the presumable asexual morph of Ohleria brasiliensis (Samuels 1980), is a member of the Ohleriaceae (Dothideomycetes) (Jaklitsch & Voglmayr 2016). Monodictys nigrosperma likely does not belong to Monodictys, but possibly to Humicola, Mycothermus or Trichocladium (Fig. 4, clade III). However, considering the unclear position of Monodictys and the low discrimination power of the LSU in the Chaetomiaceae (Wang et al., 2016a, Wang et al., 2016b), we prefer to not introduce any taxonomic change until additional sampling and molecular analyses with other markers prove the definitive placement of M. nigrosperma.
Clade IV, represented by members of Chaetosphaeriaceae (1 PP, 99 % BS), includes Chloridium chloroconium (FMR 11940), Dictyochaeta fuegiana (FMR 13126), Cacumisporium capitulatum (FMR 11339), Menispora glauca (FMR 12089), Sporoschisma mirabile (FMR 11247), and Zanclospora sp. (FMR 11585 and FMR 12186). The relationships of those genera with Chaetosphaeria and their phylogenetic position have been discussed previously by other authors (Réblová, 2000, Fernández et al., 2006). This family shows a great diversity of asexual morphs. They are mainly characterised by pigmented conidiophores and phialidic conidiogenous cells (i.e. Chloridium, Codinaea, Dictyochaeta, Gonytrichum, Menispora, Sporoschisma and Zanclospora), although genera with holoblastic conidiogenesis (i.e. Cacumisporium, Exerticlava and Stanjehughesia) or tretric conidiogenous cells (Paliphora) are also included in Chaetosphaeriaceae (Réblová, 2000, Fernández et al., 2006, Shenoy et al., 2010). Réblová et al. (2016a) recently recommended the use of the generic nomenclature attributed to the asexual morphs, such as Chloridium, Menispora and Sporoschisma, rather than the respective generic sexual names Chaetosphaeria, Zignoella or Melanochaeta.
The two isolates of Zanclospora (FMR 11584 and FMR 12186) formed a distinct and distant lineage within the Chaetosphaeriaceae. Although phylogenetic data of other Zanclospora species are not available, based on morphological criteria, Réblová et al. (1999) included this genus in the family. The two Zanclospora isolates show a very similar morphology; i.e. presence of brown, smooth, simple or branched, setiform conidiophores with phialidic conidiogenous cells that produce fusiform, hyaline and smooth-walled conidia. Since they are different from other previously described species in the genus (Calduch et al., 2002, Almeida et al., 2013), the new species Z. iberica is introduced below.
Clade V (0.99 PP, 81 % BS) includes sequences of two Helminthosphaeria species (i.e. H. clavariaurm and H. tomaculum) and one of Endophragmiella dimorphospora (FMR 12150), all representatives of the Helminthosphaeriaceae (Miller et al. 2014). This is the first time that an Endophragmiella species is linked to the Helminthosphaeriaceae based on molecular data. Previous morphological studies had associated Endophragmiella with other sexual morphs in Helminthosphaeriaceae, such as Echinosphaeria canescens and Helminthosphaeria punctata (Miller et al., 2014, Jaklitsch et al., 2016a).
The fully supported clade X includes two distinct branches, one for Spadicoides atra (EF204506) and the other for Diplococcium pulneyense (FMR 10959). Spadicoides and Diplococcium share most of their morphological characters, including brown conidiophores, terminal or intercalary, polytretic conidiogenous cells and brown conidia. They are mainly distinguished by their conidial arrangement, solitary in Spadicoides and catenate in Diplococcium. However, a previous molecular study (Shenoy et al. 2010) as well as our current analysis shows that both genera are polyphyletic. Considering that the type species of Diplococcium (D. spicatum) and Spadicoides (S. bina) are respectively placed in the Helotiales (Leotiomycetes) and the Cordanales (Sordariomycetes), our study reveals a novel phylogenetic scenario for S. atra and for D. pulneyense and D. singulare. Two new genera are introduced to accommodate S. atra and the isolate formerly identified as D. pulneyense in Hernández-Restrepo et al. (2012), i.e. Xenospadicoides and Pseudodiplococcium, respectively. Since both genera are nested in the clade X, clearly separated from any other family and order accepted in the Sordariomycetes, we introduce the new order Xenospadicoidales typified with the new family Xenospadicoidaceae for the taxonomic stability of these fungi in the class. The D. singulare clade is distantly related to other members of Sordariomycetes, forming a single lineage, paraphyletic with the Vermiculariopsiella lineage (Fig. 4, clade XIII). It is therefore considered here representative of a new genus, which is proposed as Paradiplococcium.
Clade XI (1 PP, 98 % BS) is represented by members of the genus Myrmecridium (Myrmecridiaceae, Myrmecridiales), including M. schulzeri (FMR 12424). Myrmecridium is a genus segregated from Ramichloridium, commonly found on soil and plant debris, and able to cause human and animal diseases (Arzanlou et al., 2007, De Hoog et al., 2011). It is characterised by differentiated conidiophores, with integrated sympodial and denticulate conidiogenous cells that produce pale brown conidia, often with a mucilaginous sheath (Arzanlou et al., 2007, Crous et al., 2011, Crous et al., 2015b).
Clade XIII is represented by Vermiculariopsiella spp., which includes V. pediculata (FMR 12187) isolated from Spain. Vermiculariopsiella, typified with V. immersa, is characterised by setose conidiomata, with simple or branched conidiophores bearing phialidic conidiogenous cells, often curved at the tip, that produce hyaline, aseptate conidia (Bender, 1932, Seifert et al., 2011). The genus includes more than 10 species and, based on culture methods, some of them have been described as asexual morphs of Echinosphaeria (Dhargalkar and Bhat, 2009, Jaklitsch et al., 2016a). However, the type species of Echinosphaeria, E. canescens, which has also been described producing an Endophragmiella synasexual morph (Miller et al. 2014), belongs to the Helminthosphaeriaceae (Chaetosphaeriales) as mention before. In our analysis this family is placed in a distant lineage (Fig. 4, clade V) from that composed solely of Vermiculariopsiella spp., which forms a novel strongly supported monophyletic clade in Sordariomycetes (1 PP, 98 % BS). Based on this result, we introduce a new order and new family for the genus Vermiculariopsiella.
Clade XV is represented by members of Hypocreales, and includes four of our isolates identified as Chaetopsina fulva (FMR 13129), Ch. penicillata (FMR 10948), Paramyrothecium roridum (FMR 11946) and Stachybotrys cylindrospora (FMR 11595). Some molecular studies have previously reported all these species as belonging to this order (Luo and Zhuang, 2010, Lombard et al., 2015, Lombard et al., 2016), which commonly includes asexual morphs with phialidic conidiogenous cells producing slimy conidia.
Clade XVI includes 13 of our isolates and taxa that belong to Conioscyphales, Fuscosporellales, Pleurotheciales and Savoryellales. One clade containing Parasympodiella species is shown as a novel lineage in Sordariomycetes. Furthermore, several of our isolates have also been detected as putative new species (i.e. Parasympodiella sp. FMR 13132, Conioscypha sp. FMR 13134, and the unidentified isolates FMR 11490 and FMR 12412). The Parasympodiella lineage comprised the three species P. laxa, P. eucalypti, P. elongata and our isolate FMR 13132. Parasympodiella is typified by P. laxa, and characterised by unbranched, sympodial conidiophores with thallic-arthric, terminal and intercalary conidiogenous cells that produce unbranched chains of hyaline conidia (Ponnappa 1975). Species of this genus are commonly found growing on litter. Parasympodiella sp. FMR 13132 mainly differs from the other species of the genus by its smaller conidia. It is closely related to P. elongata, but shows enough genetic difference within the LSU (97 % similarity, 619/640) and ITS (92 % similarity, 460/501) sequence data to be proposed as a new species, P. lauri. Since the monophyletic group of Parasympodiella species represents a new lineage in Sordariomycetes, we introduce the novel order Parasympodiellales, typified by the new family Parasympodiellaceae.
The Bactrodesmiastrum clade which includes B. monilioides, B. obovatum and B. pyriforme represents the recently introduced order Fuscosporellales (Yang et al. 2016).
In the additional LSU sequence analysis (Fig. 5) with a wider species sampling of four related sordariomycetous orders (i.e. Conioscyphales, Fuscosporellales Pleurotheciales and Savoryellales), the Savoryellales formed a monophyletic group, including species of Ascotaiwania, Canalisporium, Savoryella, and Triadelphia uniseptata, as previously reported in the family Savoryellaceae (Boonyuen et al., 2011, Réblová et al., 2016b). Two of our isolates, Bactrodesmium pallidum (FMR 11345) and the unidentified fungus FMR 12412 nested within this Savoryellales lineage. Bactrodesmium pallidum nested with A. mitriformis and T. uniseptata. Ascotaiwania mitriformis shows a monotosporella-like asexual morph (Ranghoo & Hyde 1998), which resembles B. pallidum and T. uniseptata in producing holoblastic, brown, septate conidia. However, B. pallidum differs from these species by its sporodochial conidiomata composed of slightly differentiated, hyaline conidiophores. The taxonomy of Bactrodesmium remains undetermined and, based on known data, it seems to be polyphyletic (Hernández-Restrepo et al. 2013). For instance, as previously published by Koukol & Kolárová (2010), B. gabretae is related with Helotiales (Leotiomycetes, Fig. 3), while Tanaka et al. (2015) reported B. cubense as a member of Massarineae, Pleosporales (Fig. 1, clade III). Bactrodesmium is one of the earliest described hyphomycete genera, with the type species B. abruptum being already described in 1865 by Berkeley & Broome from dead wood in UK (Berkeley & Broome 1865). However, the holotype was not designated in the protologue, nor authentic type material or living culture of the fungus preserved for comparison. Taking into account this fact and considering the great number of species described in the genus (ca. 50) and the restricted number of cultures available, it is challenging to reconstruct the phylogeny of Bactrodesmium. The unidentified isolate FMR 12412 grouped with A. limnetica (CBS 126576 and CBS 126792) in a fully supported clade (Fig. 5), but with a genetic difference (98 % and 95 % similarity with LSU and ITS markers, respectively) sufficient to be considered a distinct species. Considering that Ascotaiwania is polyphyletic (Boonyuen et al., 2011, Hernández-Restrepo et al., 2015a), with the type species A. lignicola placed on a separate branch far from the clade of FMR 12412 and A. limnetica, we accommodate both species in the new genus Neoascotaiwania, with the Spanish isolate being proposed as N. terrestris sp. nov.
The recently introduced Conioscyphales (Réblová et al. 2016b), typified by Conioscyphaceae, forms a well-supported lineage in our phylogenetic analyses (Fig. 4, clade XVI, 1 PP, 84 % BS; Fig. 5, 0.92 PP, 79 % BS). This includes sequences of Conioscypha varia, C. lignicola and C. peruviana retrieved from GenBank, but also sequences of Conioscypha species identified from Spanish samples, such as C. minutispora (FMR 11245, Crous et al. 2014b), C. hoehnelii (FMR 11592), and Conioscypha sp. (FMR 13134). Species of this genus are characterised by monoblastic conidiogenous cells with percurrent proliferations producing deep, hyaline collarettes and brown conidia. The singular features of Conioscypha sp. FMR 13134 (i.e. ornamented blastoconidia and a thallic-arthric synasexual morph) and its phylogenetic position justify the recognition of C. pleiomorpha sp. nov.
The isolates Phaeoisaria sparsa (FMR 11939), Sterigmatobotrys uniseptata (FMR 11937), and the unidentified fungus FMR 11490 are distributed in different well-supported lineages within the Pleurotheciales (Fig. 4, clade XVI; Fig. 5). Phaeoisaria sparsa, which is here sequenced for the first time, nested in a subclade with other Phaeoisaria species, i.e. P. clematidis, the generic type, P. fasciculata and P. sedimenticola (Fig. 5, 1 PP, 98 % BS). Phaeoisaria species are characterised by synnematous conidiomata, dark brown conidiophores, polyblastic, sympodial, denticulate conidiogenous cells and subhyaline conidia. Isolate FMR 11490 nested in another lineage with Ho. farinosum, Monotosporella setosa and Pleurothecium obovoideum (Fig. 5, 1 PP, 87 % BS). Helicoon produces hyaline conidiophores and coiled, hyaline conidia; Monotosporella has brown conidiophores bearing monoblastic, terminal conidiogenous cells with brown, septate conidia, usually obovate to pyriform. FMR 11490 resembles P. obovoideum in having unbranched, brown conidiophores, with polyblastic, denticulate conidiogenous cells and brown conidia, but it differs in having larger conidiophores and denticles, and botuliform, septate conidia. This fungus is described here as a new genus Anapleurothecium in the Pleurotheciales. Finally, S. uniseptata (FMR 11937), which is sequenced for the first time, clustered with GenBank sequences of S. macrocarpa and S. rudis (Fig. 4, 1 PP, 87 % BS; Fig. 5, 1 PP, 94 % BS). This latter species, formerly known as Taeniolella rudis, was recently transferred to Sterigmatobotrys, based on the morphology of the penicillate synasexual morph and molecular data. Taeniolella exilis, the type species, is related to the Kirschsteiniotheliaceae in Dothideomycetes (Ertz et al. 2016). The molecular taxonomy of S. macrocarpa and S. rudis has been previously studied by Réblová & Seifert (2011) and Réblová et al., 2012, Réblová et al., 2016b, who based on multi-locus phylogenies demonstrated the relationship of Sterigmatobotrys with members of Ascotaiwania, Conioscypha, Pleurotheciella and Pleurothecium.
Clade XVII (Fig. 4) is a well-supported lineage basal in the Sordariomycetes. It comprises the type species of the genus Cirrenalia, C. macrocephala, and two Spanish isolates FMR 12149 and FMR 12418 with morphological affinity to this genus. Cirrenalia macrocephala is characterised by dark sporodochial conidiomata, with conidiophores reduced to conidiogenous cells, and helicoidal brown conidia. Although the two isolates show some genetic difference, they are morphologically similar, and differ from the other species of the genus mainly by their straight conidia, as well as by the colour, size and number of septa. Based on these data, they are introduced below as C. iberica. The order affiliation of Cirrenalia remains unclear, since Abdel-Wahab et al. (2010) related the genus to the Halosphaeriales, and more recently Jaklitsch et al. (2016a) considered it as member of the Microscales.
Taxonomy
Dothideomycetes
Asterinales, Asterinaceae
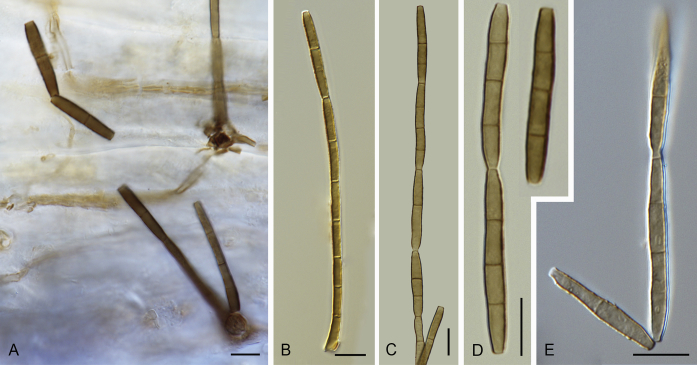
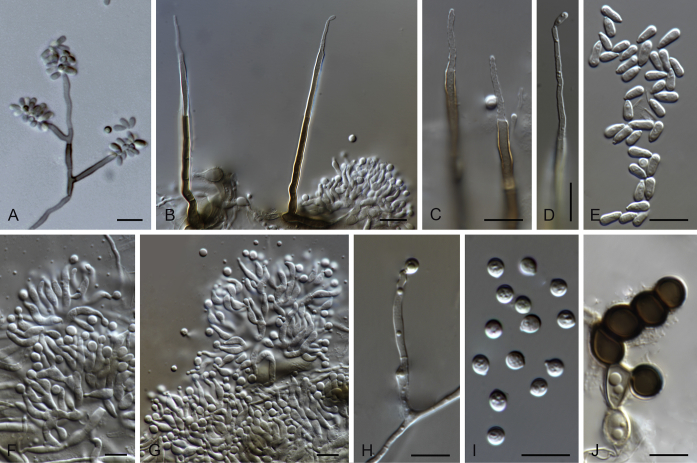
Pirozynskiella laurisilvatica Hern.-Restr., R.F. Castañeda & Gené, sp. nov. MycoBank MB820269. Fig. 6.
Fig. 6.
Pirozynskiella laurisilvatica (FMR 13133 ex-type). A, B. Conidiophores and conidia. C–E. Conidia. Scale bars = 10 μm.
Etymology: Named after the Laurisilva forest where the sample was collected, in La Gomera (Canary Islands, Spain).
Colonies on the natural substratum hairy, brown, growing on the upper leaf surface. Mycelium partly superficial and partly immersed, consisting of branched, septate, brownish, smooth- to rough-walled hyphae, 2–3 μm wide. Conidiophores semi-macronematous or micronematous, erect, cylindrical, 50–75 × 4–4.5 μm, brown, smooth-walled. Conidiogenous cells holoblastic, integrated, terminal, and cylindrical. Conidia in unbranched acropetal chains, dry, 1–3-septate, subcylindrical to cylindrical, 19–31 × 3–4 μm, tapering toward both ends 1–2 μm wide, brown to pale brown, verruculose. Sexual morph not observed.
Culture characteristics: Colonies on PCA and OA at 25 °C slow-growing, reaching 5 mm diam in 2 wk, velvety, black, margin slightly erodate; reverse black. Sporulation abundant. Conidia on OA 15–26 × 2.5–4 μm.
Specimen examined: Spain, Canary Islands, La Gomera, Garajonay Reserve Biosphere, Los Cedros, on fallen leaves of Laurus sp., Jul. 2013, M. Hernández-Restrepo & J. Guarro (holotype CBS H-21889; cultures ex-type CBS 138109, FMR 13133).
Notes: Pirozynskiella was proposed by Hughes (2007) to accommodate two asexual fungi, P. solaninum as type and P. costaricense, with simple, brown conidiophores, bearing a single unbranched acropetal chain of ellipsoidal to subcylindrical conidia. These species were segregated from the genus Heteroconium by their fungicolous nature and the sequence of septation during the maturation of conidia. The conidial septation in Pirozynskiella is at first central and then centrifugal and/or intercalary, while in Heteroconium the first formed septum is at the base of the conidium, with subsequent septa produced in sequence from the base to the apex (Hughes 2007). Pirozynskiella laurisilvatica was found growing on leaves of Laurus sp., in association with an unidentified fungus. This novel species can be easily differentiated from P. solaninum and P. costaricense by its verrucose conidia. Pirozynskiella laurisilvatica is phylogenetically related to Asterinales. However, there is no molecular data available of the other two Pirozynskiella species to confirm the phylogenetic position of the genus.
Capnodiales, Teratosphaeriaceae
Catenulostroma lignicola Hern.-Restr., J. Mena & Gené, sp. nov. MycoBank MB820270. Fig. 7.
Fig. 7.
Catenulostroma lignicola (FMR 11491 ex-type). A, B. Conidiophores and conidia. C. Conidiophores. D–N. Conidia. Scale bars = 10 μm.
Etymology: From the Latin lignum meaning wood, and colō meaning to inhabit, since this fungus was found growing on wood.
Description on OA. Mycelium mostly immersed, composed of septate, brown, smooth hyphae, 2.5–5 μm wide. Conidiophores micro- or semi-macronematous, solitary or fasciculate, erect, cylindrical, brown to dark brown, smooth to reticulate in the apex, 19.5–69.5 × 5–6 μm, or reduced to inconspicuous conidiogenous loci in the hyphae. Conidiogenous cells integrated, terminal, monoblastic, cylindrical, brown. Conidia arranged in acropetal chains, commonly unbranched, some branched near the base, tending to remain attached to each other, 0–9-septate, with different degrees of constriction at the septa, straight to slightly curved, brown to medium brown, reticulate, terminal conidia with rounded apex, truncate base, intercalary conidia with truncate ends; unicellular conidia lageniform to barrel shape, 9–13.5 × 4–5 μm; 1-septate conidia cylindrical, constricted at the septum and truncate ends, 16–25 × 4–5.5 μm; 3–9-septate, subcylindrical, 21–98.5 × 3–4.5 μm. Sexual morph not observed.
Culture characteristics: Colonies on PDA and OA at 25 °C slow-growing, reaching 6 mm diam in 2 wk, with moderate amount of mycelium, velvety, olivaceous black, margin fimbriate; reverse olivaceous black. Sporulation abundant.
Specimen examined: Spain, Galicia, Las Fragas del Eume Natural Park, on dead wood, Jul. 2010, M. Hernández-Restrepo, J. Mena & J. Guarro (holotype CBS H-22994, cultures ex-type CBS 130285, IMI 500759, FMR 11491).
Notes: Catenulostroma currently comprises six species of saprobic and pathogenic fungi. This genus was erected with C. protearum together with eight other species in Teratosphaeriaceae (Crous et al. 2007a). Later, three were added to the genus (i.e. C. eucalyptorum, C. corymbiae and C. hermanusense) (Crous et al., 2011, Crous and Groenewald, 2011), whereas four species were transferred to Neocatenulostroma (i.e. C. abietis, C. excentricum, C. germanicum and C. microsporum) (Quaedvlieg et al. 2014).
Catenulostroma lignicola is morphologically similar to C. chromoblastomycosum in having transversely septate conidia, with more than 5 septa. Nevertheless, the conidia of C. chromoblastomycosum are smaller [(8–)20–35(–60) × 4–5(–7) μm] and smooth to finely verruculose, while those in C. lignicola are larger (up to 98.5 μm) and with a reticulate ornamentation. It is noteworthy that C. chromoblastomycosum was described from a case of human chromoblastomycosis (Crous et al. 2007a).
Kirschsteiniotheliales Hern.-Restr., R.F. Castañeda, Gené & Crous, ord. nov. MycoBank MB821220.
Saprobic on wood and bark. Sexual morph. Ascomata superficial, perithecioid, dark brown to black. Hamathecium with pseudoparaphyses. Asci bitunicate, with ocular chamber. Ascospores septate, ellipsoidal, pigmented. Asexual morph. Conidiophores macronematous, brown. Conidiogenous cells blastic or tretic, brown. Conidia septate, brown.
Type family: Kirschsteiniotheliaceae Boonmee & K.D. Hyde.
Type genus: Kirschsteiniothelia D. Hawksw.
Genera included: Kirschsteiniothelia (=Dendryphiopsis), Taeniolella, Solicorynespora (based on S. insolita not generic type), Sporidesmium s.l. and Brachysporiella (based on B. navarrica not generic type).
Kirschsteiniotheliales, Incertae sedis
Brachysporiella navarrica Hern.-Restr., R.F. Castañeda & Gené, sp. nov. MycoBank MB820273. Fig. 8.
Fig. 8.
Brachysporiella navarrica (FMR 12426 ex-type). A–K. Conidiophores and conidia. L–Q. Conidia. Scale bars = 10 μm.
Etymology: Name refers to Navarra, a Spanish locality where this fungus was collected.
Description on OA. Mycelium immersed and superficial, composed of septate, straight to sinuous, brown, smooth hyphae, 2.5–4 μm wide. Conidiophores macronematous, irregularly branched, erect, cylindrical, 76–357 × 2.5–4 μm, brown, smooth. Conidiogenous cells integrated, terminal, mono- and polyblastic, cylindrical to clavate, brown, smooth-walled. Conidia solitary, 2–4-septate, covered with a mucilaginous sheath, obovoid, clavate to pyriform, 24.5–40 × 11–16 μm, with a base rounded or truncate, 2.5–4 μm wide, brown, upper cells darker, smooth, often with a portion of the conidiogenous cell attached to the base of the conidia when these are released. Sexual morph not observed.
Culture characteristics: Colonies on PDA and OA at 25 °C reaching 14 and 19 mm diam in 2 wk, respectively, with moderate amount of cottony mycelium, elevated, olivaceous black, hyaline exudate abundant, margin fimbriate; reverse olivaceous black. Sporulation abundant.
Specimen examined: Spain, Navarra, Baribar, on dead wood, Mar. 2012, M. Hernández-Restrepo & J. Capilla (holotype CBS H-22990; culture ex-type CBS 142296, FMR 12426).
Notes: Brachysporiella was introduced by Batista (1952) with B. gayana as generic type. Brachysporiella is characterised by branched or unbranched conidiophores, without basal rhizoids, and with clavate to obovoid conidia. Currently, it comprises about 12 saprobic species, which are usually found growing on wood and litter.
Brachysporiella is morphologically similar to Monotosporella; however, the taxonomy of these fungi is still unclear. Hughes (1958) introduced Monotosporella to accommodate M. setosa, a fungus characterised by unbranched conidiophores with basal rhizoids, and subglobose conidia. However, this genus was considered a synonym of Brachysporiella by Ellis (1959). Unfortunately, original material from both type species is not available for study. Réblová et al. (2016b) placed M. setosa (GenBank AF132334, isolate HKUCC 3713) in Pleurotheciales (Sordariomycetes; Fig. 4, clade XVI). Other monotosporella-like asexual morphs have been known for some Ascotaiwania species (e.g. A. mitriformis and A. sawadae in Savoryellaceae, Fig. 5) (Ranghoo and Hyde, 1998, Sivichai et al., 1998). In contrast, B. navarrica is included in an incertae sedis clade together with Solicorynespora insolita and Astrosphaeriella livistonicola, which forms a sister lineage with members of the family Kirschsteiniotheliaceae (Dothideomycetes; Fig. 1, clade V). Therefore, our phylogenetic results support the Hughes concept in considering both Brachysporiella and Monotosporella as two distinct genera. However, the taxonomic position of the former is provisional until further molecular studies with the generic type have been done.
Brachysporiella navarrica resembles B. gayana in having erect, brown conidiophores, and septate pyriform to obovoid conidia. Conidia of the novel species are 2–4-septate, slightly smaller (24.5–40 × 11–16 μm) and covered by a mucilaginous sheath, while those of B. gayana are 3-septate, larger and thicker (32–42 × 18–20 μm) without sheath (Batista 1952).
Pleosporales, Amniculicolaceae
Vargamyces aquaticus (Dudka) Tóth., Acta Mus. Silesiae, Ser. A 25(3–4): 403. 1980. Fig. 9.
Fig. 9.
Vargamyces aquaticus (A–I. FMR 11587; J–V. CBS 636.91 ex-epitype). A, E, J, K. Conidiophores and conidia. B–D. Conidiophores with percurrent proliferation (indicated by rows). F–I, L–N. Conidia. O–V. Microconidia. Scale bars = 10 μm.
Basionym: Camposporium aquaticum Dudka, Ukr. bot. Zh. 23: 91. 1966.
Synonyms: Xylomyces aquaticus (Dudka) K.D. Hyde & Goh, Mycol. Res. 103: 1573. 1999.
Sporidesmium ontariense Matsush., Matsush. Mycol. Mem. 3: 16. 1983.
Repetophragma ontariense (Matsush.) W.P. Wu, Fungal Diversity Res. Ser. 15: 82. 2005.
Description on OA. Mycelium mostly immersed, composed of septate, hyaline to pale brown, smooth hyphae, 1–3.5 μm wide. Conidiophores micronematous to semi-macronematous, solitary, erect, straight to flexuous, cylindrical, with up to 4 percurrent proliferations, 35–120 × 2.5(–4.5) μm, subhyaline to pale brown. Conidiogenous cells integrated, terminal, cylindrical to cupuliforme, 6.5–16 × 4–6 μm, hyaline to pale brown. Conidial secession rhexolytic. Conidia solitary, fusiform, 5–8-septate, truncate at the base, rounded apically brown, paler toward the ends, smooth-walled, 64–132 × 10–17 μm, base 2.5–5 μm wide. Microconidia unicellular, blastic growing on undifferentiated hyphae, solitary, terminal, lateral, or intercalary, globose to obovoid, hyaline, smooth, 2.5–4 × 1.5–2 μm. Sexual morph not observed.
Culture characteristics: Colonies on OA at 25 °C reaching 25 mm diam in 2 wk, lanose at the centre, with scarce amount of aerial mycelium toward the periphery, white to pale olivaceous buff, margin effuse, with a brick diffusible pigment; reverse saffron. Colonies on PDA at 25 °C reaching 22 mm diam in 2 wk, lanose, vinaceous buff, margin effuse and buff; reverse grey brown, buff to the periphery. Sporulation abundant on OA.
Specimens examined: Lectotype designated here: fig. 1 in Dudka I. A. New and rare species of fungi imperfecti from the basins of the southern part of Kiev Polessye. Ukrainskiy Botanichnyi Zhurnal. 1966, MBT375533. Hungary, Börzsöny Morgó stream, on submerged wood, date unknown, J. Gönczöl, MBT375360 (epitype designated here CBS H-22992, culture ex-epitype CBS 636.91). Spain, Castilla-León, Burgos, Pedroso River, on submerged wood, Nov. 2010, M. Hernández-Restrepo & J. Gené (CBS 130366, IMI 500762, FMR 11587).
Notes: In our phylogenetic tree, V. aquaticus and R. ontariense (GenBank DQ408575) formed a supported subclade in Pleosporales (Fig. 1, clade III). Vargamyces aquaticus, initially described as C. aquaticum (Dudka 1966), is commonly found on rotten submerged leaves of Alnus glutinosa, Populus nigra and Acer sp. (Révay et al. 2014). Sporidesmium ontariense was introduced by Matsushima (1983) for a fungus that was found in Canada, growing on a dead branch of Aceris sacchari. It was later transferred to Repetophragma because the conidiophores were shown to proliferate percurrently (Wu & Zhuang 2005). Although similarities among V. aquaticus and R. ontariense have been noticed previously (Gönczöl et al., 1990, Révay et al., 2014), no new combination has been introduced. Morphological and molecular data suggest that R. ontariense and V. aquaticus are conspecific, and here we list them as synonyms. Based on LSU sequences of R. ontariense (DQ408575, culture HKUCC 10830), Zhang et al. (2009a) placed this species in the Amniculicolaceae.
The type material for V. aquaticus has been lost (Révay et al. 2014); therefore, the illustration included in the protologue (Dudka 1966) is selected as lectotype. In addition, to assure the availability of information for modern identification, CBS 636.91 is designated as ex-epitype culture. Morphological features of the epitype fit well with the protologue of C. aquaticum (Dudka 1966). The Spanish strain shows slightly larger and more septate conidia than those described above (101–135 × 16–20 μm, base 4–6 μm wide, 5–9-septate). However, CBS 636.91 and FMR 11587 show identical LSU and ITS sequences.
Melanommataceae
Pleotrichocladium Hern.-Restr., R.F. Castañeda & Gené, gen. nov. MycoBank MB820277.
Etymology: Pleo- referring to Pleosporales; and -trichocladium referring to the asexual genus Trichocladium. Morphologically similar to Trichocladium, but phylogenetically related to Pleosporales.
Mycelium superficial and immersed, composed of branched, septate, hyaline to pale brown, smooth hyphae. Conidiophores micronematous, reduced to a hyphal cell that extents laterally to form a conidium or arising as short lateral pedicels on the hyphae, unbranched or loosely branched. Conidiogenous cells integrated, mono- and polyblastic, cylindrical, subglobose or barrel-shaped, subhyaline to pale brown, smooth. Conidial secession schizolytic. Conidia solitary, septate, ovoid, ellipsoid to clavate, straight or curved, brown and smooth. Sexual morph not observed.
Type species: Pleotrichocladium opacum (Corda) Hern.-Restr., R.F. Castañeda & Gené.
Pleotrichocladium opacum (Corda) Hern.-Restr., R.F. Castañeda & Gené, comb. nov. MycoBank MB820278. Fig. 10.
Fig. 10.
Pleotrichocladium opacum (FMR 12416 ex-epitype). A–J. Conidiophores and conidia. Scale bars = 10 μm.
Basionym: Sporidesmium opacum Corda Icon. Fung. 1: 7. 1837.
Synonyms: Xenodochus opacus (Corda) Bonord., Handb. Allgem. mykol.: 49. 1851.
Clasterosporium opacum (Corda) Sacc., Syll. Fung. 4: 387. 1886.
Trichocladium opacum (Corda) S. Hughes, Trans. Br. Mycol. Soc. 35: 154. 1952.
Description on OA. Mycelium partly superficial and partly immersed, composed of septate, hyaline to pale brown, smooth hyphae, 1.5–3 μm wide. Conidiophores micronematous, often reduced to a hyphal cell that laterally extents to form a conidium or arising as short lateral pedicels on the hyphae, unbranched or loosely branched. Conidiogenous cells integrated, mono- and polyblastic, terminal or intercalary, cylindrical or doliiform, 7–14 × 3–3.5 μm, subhyaline to pale brown, smooth. Conidia solitary, 2–4(–5)-septate, ovoid, ellipsoid or clavate, 22–37 × 12–18.5 μm, dark brown, basal cells paler, smooth. Sexual morph not observed.
Culture characteristics: Colonies on OA at 25 °C reaching 35–55 mm diam in 2 wk, lanose, white to grey olivaceous, margin white, effuse; reverse olivaceous black. Colonies on PDA at 25 °C reaching 35–40 mm diam in 2 wk, lanose, smoke grey, grey olivaceous or greenish olivaceous, margin white, effuse; reverse olivaceous black. Sporulation moderate to abundant.
Specimens examined: Lectotype designated here: tab. II, fig. 115 in Corda ACJ, Icones Fungorum hucusque Cognitorum 1: i–iv, 1837. MBT375536. Antarctica, King George, Jubany, on lichen, 1991, C. Möller (CBS 709.92). Austria, Vorarlberg, isolated from soil, summer 1966, M.A.A. Schipper (CBS 534.66). The Netherlands, Baarn, garden Eemnesserweg 90, on dead wood of Thuja occidentalis, Mar 1970, H.A. van der Aa (CBS 450.70). Spain, Aragón, Ordesa y Monte Perdido, National Park, isolated from soil, Mar. 2011, M. Hernández-Restrepo & J. Capilla (CBS 142288, FMR 12088). Navarra, Robledal de Orgi, on dead wood, Mar. 2012, M. Hernández-Restrepo & J. Gené, MBT375363 (epitype designated here CBS H-22985; cultures ex-epitype CBS 142294, FMR 12416).
Notes: Trichocladium opacum is a widely-distributed species, usually found on plant material or isolated from soil (Kendrick and Bhatt, 1966, Ellis, 1971). Corda (1837) introduced that species as S. opacum from dead wood in Reichenberg (Czech Republic). Later it was re-described and illustrated by Hughes (1952), and considered congeneric with T. asperum. Holotype material is unavailable for S. opacum. However, the protologue of the species contains an illustration and is designated here as the lectotype of S. opacum. Furthermore, an ex-epitype culture is selected to fix the use of this name. It is noteworthy that Hughes (1958) considered more Sporidesmium species conspecific with T. opacum, i.e. S. ovoideum, S. fasciculare and S. pyriforme. However, further studies based on type specimens are needed to confirm these synonyms.
In our phylogenetic analyses, several strains of T. opacum were placed in Melanommataceae (Pleosporales; Fig. 1, clade III), as previously suggested by Mantle et al. (2006). Since Trichocladium is polyphyletic with the type species, T. asperum, placed in the Chaetomiaceae (Hambleton et al. 2005) (Sordariales; Fig. 4, clade III), we propose the new genus Pleotrichocladium to accommodate T. opacum. Besides the phylogenetic differences, P. opacum differs morphologically from T. asperum by its pale brown conidiogenous cells and smooth conidia with schizolytic secession. Trichocladium asperum has hyaline conidiogenous cells, and its conidia are warty and with rhexolytic secession (Fig. 22).
Fig. 22.
Trichocladium asperum (CBS 903.85 ex-epitype). A–F. Conidiophores and conidia. G–J. Conidia. Scale bars = 10 μm.
Pleomonodictydaceae Hern.-Restr., J. Mena & Gené, fam. nov. MycoBank MB820279.
Saprobic on wood and bark. Sexual morph. Unknown. Asexual morph. Conidiophores micro- to semi-macronematous, often reduced to conidiogenous loci in the hyphae. Conidia blastic, solitary or in short chains, variable in shape, muriform, dark brown to black, verrucose to tuberculate.
Type genus: Pleomonodictys Hern.-Restr., J. Mena & Gené.
Included genus: Pleomonodictys.
Notes: In a multi-locus study using LSU, SSU and tef1 genes, Tanaka et al. (2015) showed that Monodictys capensis clustered together with Inflatispora pseudostromatica in a clade of uncertain position “unknown clade IV” in the suborder Massarineae (Pleosporales). The phylogeny of the former species was based on the reference strain CBS 134928, identified from dead wood in Russia (Mel'nik & Shabunin 2010), not from the type material. In our phylogenetic analysis that isolate was shown to be closely related to Monodictys sp. FMR 12716, which formed a lineage very distant to that of I. pseudostromatica (Fig. 1, Clade III). Therefore, the taxonomy of these two monodictys-like fungi is resolved with the introduction of Pleomonodictydaceae to accommodate the new genus Pleomonodictys, although the family placement of Inflatispora remains unclear.
Pleomonodictys Hern.-Restr., J. Mena & Gené, gen. nov. MycoBank MB820280.
Etymology: Pleo- referring to Pleosporales; and -monodictys referring to the asexual genus Monodictys. Morphologically similar to Monodictys, but phylogenetically related to Pleosporales.
Colonies effuse, black. Mycelium mostly immersed, composed of branched, septate, smooth often verruculose hyphae. Conidiophores micronematous or semi-macronematous, often reduced to conidiogenous loci on the hyphae. Conidia blastic, solitary or in short chains, variable in shape, muriform, dark brown to black, verrucose to tuberculate. Sexual morph not observed.
Type species: Pleomonodictys descalsii Hern.-Restr., J. Mena & Gené.
Notes: Pleomonodictys is introduced for P. descalsii and P. capensis, previously accommodated in Monodictys. Those fungi differ morphologically from M. putredinis, the type species of Monodictys, in having verrucose to tuberculate conidia and/or hyphae. Monodictys is a polyphyletic genus with species evenly spread in different classes, i.e. Dothideomycetes, Sordariomycetes and Leotiomycetes (Campbell et al., 2002, Han et al., 2014, Tanaka et al., 2015). Although molecular data for the type species of Monodictys are not available, based on culture methods, M. putredinis has been reported as the asexual morph of Ohleria brasiliensis (Samuels 1980). Recently, Ohleria was included in the pleosporalean family Ohleriaceae (Jaklitsch & Voglmayr 2016). Phylogenetic reassessment of species in Monodictys and related sexual genera, including types, is needed to clarify the taxonomy of this genus.
Pleomonodictys capensis (R.C. Sinclair et al.) Hern.-Restr., J. Mena & Gené, comb. nov. MycoBank MB821221.
Basionym: Monodictys capensis R.C. Sinclair et al., Mycotaxon 59: 359. 1996.
Description and illustration: Sinclair et al. (1996)
Specimen examined: South Africa, Cape Province, on decorticated wood, Jul. 1994, R.C. Sinclair (ex-type cultures CBS 968.97, PPRI 5984).
Notes: Monodictys capensis was mainly characterised by the irregularity in the conidial shape and ornamented wall (Sinclair et al. 1996). The ITS sequence of the ex-type strain CBS 968.97 and that retrieved from GenBank (LC014570) corresponding to Mel'nik's strain (CBS 134928) were identical, confirming the identification of this latter strain.
Pleomonodictys descalsii Hern.-Restr., J. Mena & Gené, sp. nov. MycoBank MB820281. Fig. 11.
Fig. 11.
Pleomonodictys descalsii (FMR 12716 ex-type). A–L. Conidiophores and conidia. M–U. Conidia. Scale bars = 10 μm.
Etymology: In honour to the Spanish mycologist Enrique Descals.
Colonies on OA. Mycelium partly superficial and partly immersed, composed of branched, septate, brown to pale brown, nodulose, smooth hyphae, 2.5–5 μm wide. Conidiophores micronematous, often reduced to conidiogenous loci in the hyphae, terminal or intercalary. Conidia solitary or in irregular branched chains, muriform, obovoid, clavate to pyriform, ellipsoid to subglobose, 28–70 × 24–54 μm, brown to dark brown, or irregularly pigmented, sometimes basal cells paler than the others, base rounded or truncate, 3–8.5 μm wide, tuberculate. Sexual morph not observed.
Culture characteristics: Colonies on PDA and OA at 25 °C reaching 10 mm diam in 2 wk, with moderate amount of aerial mycelium, white, fasciculate, immersed mycelium iron grey, margin fimbriate or effuse on PDA and OA, respectively; reverse olivaceous grey in PDA, pale mouse grey in OA. Sporulation abundant.
Specimen examined: Spain, Mallorca, Sierra de Tramuntana, on bark of Quercus sp., Sep. 2012, M. Hernández-Restrepo, E. Descals & J. Gené (holotype CBS H-22991, cultures ex-type CBS 142298, FMR 12716).
Notes: This new species differs from P. capensis in having smaller conidia (P. capensis 30–100 × 17–60 μm from the natural substratum, up to 175 × 110 μm in culture). Monodictys castaneae (Ellis 1971) resembles the novel species in having ornamented conidia. However, hyphae in M. castanaea are smooth and its conidia smaller (14–40 × 10–25 μm). The phylogenetic placement of M. castaneae is still unknown, but unpublished LSU and ITS sequences of three strains (CBS 100.07, CBS 101.60 and CBS 102.60, not ex-types) relate this Monodictys species with Preussia (Sporomiaceae, Pleosporales).
Venturiales, Venturiaceae
Magnohelicospora fuscospora (Linder) R.F. Castañeda, Hern.-Restr. & Gené, comb. nov. MycoBank MB820282.
Basionym: Helicoon fuscosporum Linder, Annls Miss. Bot. Gdn. 16: 326. 1929.
Notes: LSU analyses of the sequences of M. iberica and Ho. fuscosporum, the latter retrieved from GenBank and generated by Tsui & Berbee (2006), revealed that both fungi are congeneric (Fig. 1, clade I). Therefore, based on their phylogeny and morphological affinities, we transfer Ho. fuscosporum to Magnohelicospora. These two species differ in conidial size; while M. iberica has conidia 25–50 × 17–30 μm (up to 60 μm long in culture), in M. fuscospora they are 20–25 × 22.5–33 μm (Linder 1929).
Magnohelicospora iberica R.F. Castañeda et al., Mycotaxon 121: 172. 2013. Fig. 12.
Fig. 12.
Magnohelicospora iberica (FMR 12414 ex-epitype). A–C. Conidiophores and conidia. D. Conidiogenous cells. E–L. Conidia. Scale bars = 10 μm.
Description on OA. Mycelium partly superficial and partly immersed, consisting of septate, branched, sometimes sinuous, brown, smooth hyphae, 1.5–3.5 μm wide. Conidiophores erect, unbranched, brown, smooth, cylindrical, 24–60 × 2.5–4 μm. Conidiogenous cells mono- and polyblastic, cylindrical, 10–22 × 2.5–3.5 μm, denticulate; denticles 1–4 × 1.5–3 μm. Conidial secession schizolytic. Conidia dry, solitary, compactly circinate in three dimensions, doliiform to somewhat conical, 25–60 × 17–27 μm, composed of a filament tightly coiled 7–10 times in 3-dimensions, euseptate, brown or olivaceous-brown, smooth, 3–4 μm wide. Sexual morph not observed.
Culture characteristics: Colonies on PDA and OA at 25 °C, 18–20 mm diam after 2 wk, elevated, velvety, with moderate amount of short mycelium, mouse grey, margin fimbriate, greyish sepia; reverse dark mouse grey. Sporulation moderate.
Specimen examined: Portugal, Minho province, Lagoas do Bertiandos protected area, on rotten leaf of unidentified plant, Nov. 2011, R.F. Castañeda, M. Hernández-Restrepo, J. Gené & J. Mariné-Gené (holotype, HAL 2447 F; isotype, FMR 12184). Spain, Navarra, Robledal de Orgi, on dead leaves, Mar. 2012, M. Hernández-Restrepo & J. Capilla, MBT-375365 (epitype designated here CBS H-22989; culture ex-epitype CBS 142293, FMR 12414).
Notes: Magnohelicospora iberica was described from Portugal growing on dead leaves, but the type specimen could not be cultivated (Castañeda-Ruiz et al. 2012). In contrast, the second isolate of the species from Spain, which morphologically fits with the protologue on the natural substratum, grew well in the different culture media tested. However, it produced conidia slightly longer and thinner than those described in the holotype (25–60 × 17–27 μm vs. 35–50 × 23–30 μm).
Dothideomycetes, Incertae sedis
Oncopodiella trigonella (Sacc.) Rifai 1965, Persoonia 3: 409. Fig. 13.
Fig. 13.
Oncopodiella trigonella (FMR 10788 ex-epitype). A–G. Conidiophores and conidia. H–S. Conidia. Scale bars = 10 μm.
Basionym: Sporidesmium trigonellum Sacc., Michelia 2 (no. 8): 641. 1882.
Description on OA. Mycelium mostly immersed, composed of septate, hyaline to brown, smooth hyphae, 1.5–2 μm wide. Conidiophores macronematous or reduced to conidiogenous cells. Conidiogenous cells mono- and polyblastic, sympodial, flexuous, cylindrical to conical, 5–27 × 1.5–2.5 μm, hyaline to pale brown. Conidia solitary, muriform, obovoid to oval, corniculate, 17–23.5 × 10.5–16.5 μm, with 2–4, mostly 3, hyaline horn-like protruding cells, 2.5–5 × 3–5.5 μm, basal cell conical and truncate, 1.5–2 μm wide, at the begin hyaline becoming brown with the age, smooth. Sexual morph not observed.
Specimens examined: Belgium, Malmedy, on bark of Ailanthus, holotype PAD Libert. 432. Spain, Teruel province, Valbona, on bark of unidentified tree, Oct. 2009, col. M. Hernández-Restrepo, MBT375358 (epitype designated here CBS H-22993; cultures ex-epitype CBS 126413, MUCL 52643, FMR 10788).
Notes: This isolate fits in all morphological aspects with the protologue of O. trigonella (Saccardo, 1882, Rifai, 1965), the type species of Oncopodiella. The genus currently comprises more than 10 species (Magyar & Révay 2009), but no molecular data are available to assess their relationship. Here, we propose CBS 126413 as ex-epitype of O. trigonella to fix the concept of both the species and genus. Further taxon sampling is needed to determine the real taxonomic structure of this undescribed lineage within the Dothideomycetes.
Leotiomycetes
Helotiales
Bloxamiaceae Locq., fam. nov. MycoBank MB820283.
Synonym: Bloxamiaceae Locq., Mycol. gén. struct. (Paris): 209. 1984. nom. inval. (Art. 39.1).
Saprobic on wood. Sexual morph. Unknown. Asexual morph. Conidiomata sporodochial, pulvinate with a basal stroma. Conidiophores often reduced to conidiogenous cells. Conidiogenous cells phialidic, terminal arising from the stroma surface in a densely-packed palisade, subcylindrical, pale brown, smooth. Conidia produced in easily fragmenting basipetal chains, cylindrical to quadrate, truncate, hyaline, smooth.
Type genus: Bloxamia Berk. & Broome.
Included genus: Bloxamia.
Notes: According to MycoBank and Index Fungorum, Bloxamiaceae is invalid because a Latin diagnosis was not provided by the author (Locquin 1984) (Art. 39.1, Melbourne). Here we validate this name by providing a valid description. In our phylogenetic tree of Leotiomycetes (Fig. 3) this family is represented by a single strain of the type species, Bloxamia truncata.
Leptodontidiaceae Hern.-Restr., Crous & Gené, fam. nov. MycoBank MB820284.
Colonies growing moderately slowly, appearing smooth to funiculose, grey to black or yellow. Asexual morph. Conidiophores erect, brown, paler at the apex, simple or irregularly branched. Conidiogenous cells polyblastic, integrated, terminal, cylindrical to lageniform, with pale brown venter, and a hyaline rachis with inconspicuous scars. Conidia dry, solitary, unicellular, subcylindrical to narrowly obovate, straight or slightly curved, hyaline, with truncate base. Synasexual morph beauveria-like. Conidiophores macronematous, frequently in groups or dense clusters, or reduced to conidiogenous cells, hyaline. Conidiogenous cells polyblastic, sympodial, lageniform to subcylindrical, curved, hyaline. Conidia in slimy masses, unicellular, globose to subglobose, with apiculate base, guttulate, hyaline, smooth. Chlamydospores terminal and intercalary, solitary or in simple or branched chains, ellipsoidal to subglobose, hyaline becoming brown with the age, smooth. Sexual morph unknown.
Type genus: Leptodontidium de Hoog.
Included genus: Leptodontidium.
Notes: Leptodontidiaceae is hereby introduced to accommodate species of Leptodontidium. This genus currently comprises about nine species (De Hoog and Hermanides-Nijhof, 1977, Castañeda-Ruiz, 1988, Baral, 2015). In our phylogenetic tree (Fig. 3), this family is represented by three taxa, namely L. trabinellum (generic type), L. irregulare and a new species from Spain, L. aureum. The relationships with other species of the genus need further molecular analyses.
Leptodontidium aureum Hern.-Restr., Guarro & Gené, sp. nov. MycoBank MB820285. Fig. 14.
Fig. 14.
Leptodontidium aureum (FMR 11834 ex-type). A–E. Asexual morph. A, B. Conidiophores. C, D. Conidiogenous cells. E. Conidia. F–I. Synasexual morph. B, F–H. Conidiophores, conidiogenous cells and conidia. I. Conidia. J. Chlamydospores. Scale bars = 10 μm.
Etymology: From the Latin -aureus, meaning yellow; referring to the colour of the pigment produced in culture.
Description on OA. Mycelium partly immersed and partly superficial, composed of septate, hyaline to brown, smooth hyphae, 2.5–4 μm wide. Asexual morph. Conidiophores macronematous, erect, straight, simple or irregularly branched, 37–108 × 2–4.5 μm, brown at the base, paler at the apex, smooth. Conidiogenous cells polyblastic, integrated, terminal, sympodial, cylindrical to lageniform, 4.5–51 × 1–3.5 μm, with pale brown venter, and a hyaline rachis often with a terminal conidium remaining attached; rachis acicular, 1–30 × 1–1.5(–2) μm, provided with minute, crowded, unpigmented conidial scars. Conidia dry, solitary, obovoid to oblong, 4.5–8 × 2–3 μm, truncate base, hyaline, smooth. Synasexual morph beauveria-like. Conidiophores macronematous, grouped in dense clusters, hyaline. Conidiogenous cells polyblastic, sympodial, lageniform to subcylindrical, curved, 5–14.5 × 2.5–3 μm, apex 1–1.5 μm long, hyaline, smooth. Conidia in buff colour slimy masses, globose, 3–4 μm diam, with apiculate base, guttulate, hyaline, smooth. Chlamydospores terminal or intercalary, solitary or in simple or branched chains, 0–1-septate, ellipsoidal to subglobose, 6–19 × 4–7.5 μm, hyaline becoming brown, smooth. Sexual morph not observed.
Culture characteristics: On PDA at 25 °C reaching 23 mm diam in 2 wk, flat or slightly elevated at the centre, funiculose, dark mouse grey with some slimy buff masses, aerial mycelium white to mouse grey, diffusible pigment at first (1 wk) orange becoming reddish (2 wk), margin whitish, effuse; reverse zonate, with concentric areas, from orange at the centre to dark brown and white toward the periphery. On OA at 25 °C reaching 26 mm diam in 2 wk, flat, sparse aerial mycelium, somewhat velvety, slimy at the centre, luteous, margin whitish effuse; reverse luteous. Sporulation abundant of both asexual morphs on the two media tested.
Specimen examined: Spain, Galicia, Fragas do Eume Natural Park, isolated from forest soil, May 2010, M. Hernandez-Restrepo, J. Mena-Portales & J. Guarro (holotype CBS H-22997; cultures ex-type CBS 142316, FMR 11834).
Notes: Leptodontidium aureum is morphologically similar to the generic type, L. trabinellum, in having erect conidiophores and conidiogenous cells with a long rachis; nevertheless, conidia of the latter are cylindrical, straight to curved, and smaller (3.5–5 × 1–1.5 μm) than those of L. aureum, which are obovoid to oblong and 4.5–8 × 2–3 μm. Leptodontidium irregulare is the closest relative of L. aureum, from which it differs by its larger beauveria-like conidia (up to 5.5 μm diam vs. 3–4 μm in L. aureum) and by the smaller chlamydospores (8–13 × 4–6 μm vs. 6–19 × 4–7.5 μm in L. aureum). Furthermore, the ITS sequence of L. aureum is 90 % (301/306) similar to that of the ex-type strain (CBS 851.73) of L. irregulare and 87 % (287/330) to that of the ex-type strain (CBS 329.53) of L. trabinellum, respectively.
Mollisiaceae
Fuscosclera Hern.-Restr., J. Mena & Gené, gen. nov. MycoBank MB820286.
Etymology: Latin -fusco, meaning dark; and Greek -sclera, meaning hard; referring to the dark brown to black multiseptate conidia.
Mycelium partly superficial and partly immersed, composed of cylindrical, dark brown, septate hyphae, often aggregated in strands. Conidiophores semi-macronematous or micronematous, brown. Conidiogenous cells terminal or intercalary, blastic, meristematic. Conidia consisting of multiseptate, irregular and dark brown to black propagules formed by masses of rounded to angular cells. Sexual morph unknown.
Type species: Fuscosclera lignicola Hern.-Restr., J. Mena & Gené.
Fuscosclera lignicola Hern.-Restr., J. Mena & Gené, sp. nov. MycoBank MB820287. Fig. 15.
Fig. 15.
Fuscosclera lignicola (FMR 11236 ex-type). A–Q. Hyphae, conidiophores and conidia. R–T. Conidia. Scale bars = 10 μm.
Etymology: From the Latin lignum meaning wood, and colō meaning to inhabit, since this fungus was found growing on wood.
Description on OA. Mycelium immersed and superficial, composed of septate, dark brown, cylindrical hyphae, 2–5 μm wide, often aggregated in strands of 2–4 hyphae. Conidiophores single, unbranched, septate, arising from the aerial mycelium, up to 36 μm long and 2–5 μm wide, often reduced to conidiogenous loci on the hyphae, brown. Conidia consisting of multiseptate, dark brown to black, irregular propagules, 14–31.5 × 11–38 μm, formed by masses of rounded to angular cells, 5–8 μm wide. Sexual morph not observed.
Culture characteristics: Colonies on PDA at 25 °C reaching 24 mm diam in 2 wk, elevated, with dense funiculose mycelium at the centre, cottony to the periphery, mouse grey, margin whitish, effuse to fimbriate; reverse dark mouse grey. Colonies on OA at 25 °C reaching 26 mm diam in 2 wk, elevated, funiculose centre, velvety to the periphery, metallic dark olivaceous, margin whitish, effuse to fimbriate; reverse dark olivaceous. Sporulation abundant.
Specimen examined: Spain, Galicia, Los Ancares Natural Park, on dead wood, Oct. 2010, M. Hernández-Restrepo, J. Mena-Portales & J. Guarro (holotype CBS H-22996; cultures ex-type CBS 142287, FMR 11236).
Notes: Meristematic fungi are scattered in different orders in Ascomycota, but mainly placed in Dothideomycetes (Selbmann et al., 2005, Egidi et al., 2014). Fuscosclera is related to Mollisiaceae (Helotiales, Leotiomycetes) and is distinguished from other meristematic fungi by its single, multi-celled brown conidia. Trimmatostroma is another meristematic fungus member of Mollisiaceae, linked with Mollisia (Crous et al. 2007a). Nevertheless, Fuscosclera is easily differentiated from Trimmatostroma salicis, the generic type, by its solitary conidia consisting of irregular masses of cells, while the conidia of Trimmatostroma are cylindrical, transversely septate, and produced in branched chains (Ellis 1971).
Lauriomycetales Hern.-Restr., R.F. Castañeda & Guarro, ord. nov. MycoBank MB820288.
Saprobic on dead fallen leaves. Sexual morph. Unknown. Asexual morph. Conidiophores macronematous, mononematous, developing a complex, branched conidiogenous apparatus at the apex. Conidiogenous cells blastic, discrete, hyaline. Ramoconidia in 1 or several tiers, hyaline, smooth. Conidia in acropetal chains, hyaline, smooth.
Type family: Lauriomycetaceae Hern.-Restr., R.F. Castañeda & Guarro.
Lauriomycetaceae Hern.-Restr., R.F. Castañeda & Guarro, fam. nov. MycoBank MB820289.
Conidiophores macronematous, mononematous, erect, straight, septate, cylindrical, brown paler at the apex, developing a complex, branched conidiogenous apparatus at the apex. Conidiogenous cells blastic, discrete, terminal, hyaline. Conidial secession schizolytic. Ramoconidia in 1 or several tiers, hyaline, smooth. Conidia in acropetal chains, hyaline, smooth.
Type genus: Lauriomyces R.F. Castañeda.
Included genus: Lauriomyces.
Notes: Lauriomycetales comprises only Lauriomyces. This genus was introduced with L. pulcher as generic type together with other three species, L. catenata, L. helicocephala and L. ventricosa (Castañeda-Ruiz & Kendrick 1990). Currently, Lauriomyces comprises about nine species often found on fallen leaves. Morphological similarities with Haplographium (currently Dematioscypha) were previously discussed by other authors (Castañeda-Ruiz and Kendrick, 1990, Somrithipol and Jones, 2007). However, our phylogenetic analysis supports that Haplographium and Lauriomyces are different genera and they comprise two distant monophyletic lineages in Leotiomycetes.
Sordariomycetes
Chaetosphaeriales, Chaetosphaeriaceae
Zanclospora iberica Hern.-Restr., J. Mena & Gené, sp. nov. MycoBank MB820290. Fig. 16.
Fig. 16.
Zanclospora iberica (FMR 11584 ex-type). A, B. Conidiophores. D–I. Conidiogenous cells. J. Conidia. Scale bars = 10 μm.
Etymology: Referred to the geographical origin where the fungus was found, the Iberian Peninsula.
Description on OA. Mycelium partly immersed and partly superficial, composed of branched, septate, hyaline and brown, smooth hyphae, 1.5–3 μm wide. Conidiophores macronematous, erect, straight, sometimes curved, setiform, attenuate toward the apex, simple or irregularly branched, 148–340 μm long, 3–6 μm wide at the base, 6–10 μm wide at the fertile region, 2.5–5 μm wide at the apex, brown paler in the apex, smooth; fertile region of the conidiophore situated about 43–194 μm below the apex. Conidiogenous cells discrete, monophialidic, formed in 1–3 whorls, appressed to the conidiophore laterally, lageniform to ampulliform, 8.5–12.5 × 4–6.5 μm, 1–2(–2.5) μm wide at the tapered open distal end, subhyaline to pale brown, smooth. Conidia unicellular, fusiform, falcate, straight or slightly curved, 12.5–22 × 2–3 μm, rounded end, hyaline, smooth.
Culture characteristics: Colonies on PCA at 25 °C reaching 4–7 mm diam in 2 wk, velvety, brown, margin effuse; reverse black. OA at 25 °C reaching 5–7 mm diam in 2 wk, elevated, velvety to funiculose, black, margin effuse; reverse similar. Sporulation abundant.
Specimens examined: Spain, Asturias, Picos de Europa National Park, La Molina, on dead wood of unidentified plant, Jul. 2010, M. Hernández-Restrepo, J. Mena-Portales & J. Guarro (holotype CBS H-22995; cultures ex-type CBS 130426, FMR 11584). Portugal, Minho Province, Lagoas do Bertiandos protected area, on dead wood of unidentified plant, Nov. 2011, R.F. Castañeda, M. Hernández-Restrepo, J. Gené & J. Mariné-Gené (FMR 12186).
Notes: Zanclospora iberica resembles Z. novae-zealandiae, the generic type, in having branched conidiophores. Zanclospora novae-zealandiae is characterised by apically verrucose conidiophores, conidiogenous cells are arranged in whorls of 3–7 tiers, and conidia are larger and 18–35 × 1.6–2.5 μm (Hughes & Kendrick 1965). In contrast, the conidiophores in Z. iberica are smooth, its conidiogenous cells are arranged in whorls of 1–3 tiers, and the conidia are 12.5–22 × 2–3 μm. Unfortunately, there are no sequences available from Z. novae-zealandiae for comparison. This is the first time that the placement of Zanclospora is confirmed in Chaetosphaeriaceae.
Conioscyphales, Conioscyphaceae
Conioscypha pleiomorpha Hern.-Restr., R.F. Castañeda & Gené, sp. nov. MycoBank MB820291. Fig. 17.
Fig. 17.
Conioscypha pleiomorpha (FMR 13134 ex-type). A–C. Conidiogenous cells and conidia. D. Thallic synasexual morph conidiophores and conidia. Scale bars = 10 μm.
Etymology: Greek, pleio-, meaning more than usual; and -morpha, referring to existing two different forms of conidial ontogeny.
Colonies on the natural substratum effuse, black. Mycelium mostly immersed, composed of branched, septate, hyaline, smooth hyphae, 1–3 μm wide. Conidiophores micronematous, reduced to conidiogenous cells. Conidiogenous cells monoblastic, cupulate, endogenous, multilayer-cupulate collarette after several percurrent enteroblastic tiny elongations, 9–12 × 13–16 μm, up to 14 μm deep, hyaline or subhyaline, smooth. Conidia solitary, unicellular, ellipsoidal, obovoid or subglobose, 13–18 × 12–14 μm, base truncate with a central pore of 1–1.5 μm diam, brown, pitted.
Culture characteristics: Colonies on PCA and OA at 25 °C reaching 10 mm diam in 2 wk, scarce aerial mycelium, powdery, elevate, black, margin erodate, white; reverse grey. Sporulation abundant. Mycelium composed of septate, subhyaline to very pale brown, smooth hyphae, 1–4 μm wide. Conidiogenous cells similar to those observed on the natural substratum but measuring 12–18 × 8–10 μm, up to 14 μm deep. Conidia subglobose to broadly ellipsoidal or broadly obovoid to elongate napiform, sometimes slightly curved toward the base, 9–15 × 6–9 μm, truncate base with a central pore of 1–1.5 μm diam, usually with strongly pigmented deposit with lacunose aspect, dark reddish-brown to black, smooth. Synasexual morph consisting of thallic-arthric conidia formed by disarticulation of branched or unbranched conidiogenous hyphae on the aerial mycelium; apical conidia oblong with rounded apex and truncate base, intercalary and basal conidia doliiform, sub-hemispherical, or Y-shaped, terminally truncated, 3–5 × 2–4 μm, light brown to brown, smooth.
Specimen examined: Spain, Canary Islands, Tenerife, Las Mercedes, on dead wood of unidentified plant, Jul. 2013, M. Hernández-Restrepo & J. Guarro (holotype CBS H-21890; cultures ex-type FMR 13134, CBS 138110).
Notes: Phylogenetically, C. pleiomorpha formed a separate branch, basal to the clade of other species of Conioscypha. It is noteworthy that in culture this species produces a synasexual morph with thallic-arthric conidia similar to that observed in C. dimorpha (Matsushima 1996). However, the synasexual morph of this latter was described by the author as “microconidia oblong to cylindrical rounded at the apex and truncate at the base, 2–3 × 2–2.5 μm”, without any mention on the mode of conidial ontogeny. The blastic conidia of C. dimorpha are oblong to cylindrical, (8–)10–14(–18) × (4–)4.5–5.5(–6.5) μm, and smooth-walled, and can be clearly differentiated from those of C. pleiomorpha which are much wider (13–18 × 12–14 μm), and pitted on the natural substratum.
Microascales, Halosphaeriaceae
Cirrenalia iberica Hern.-Restr. & Gené, sp. nov. MycoBank MB820292. Fig. 18.
Fig. 18.
Cirrenalia iberica (FMR 12149 ex-type). A–H. Conidiophores and conidia. I–M. Conidia. Scale bars = 10 μm.
Etymology: Refers to the name of the region, Iberian Peninsula, from which the species was collected.
Description on OA. Mycelium immersed and superficial, composed of septate, branched, hyaline, smooth hyphae, 1–4 μm wide. Conidiophores micronematous, pale brown to brown. Conidiogenous cells mono- or polyblastic, integrated, clavate, cylindrical, 11–23 × 4.5–8.5 μm, base 2–3.5 μm wide, pale brown to brown, smooth. Conidia solitary, straight or slightly curved, (1–)2–3(–4)-septate, constricted at the septa, 19–41 μm long, cells increasing in size and pigmentation from the base to the apex; basal cell, subglobose, hemi-globose to cuneiform, often paler than the rest, 3–9(–12.5) μm wide; median cell subglobose, mid brown; apical cell subglobose, 10–15 μm wide, mid brown.
Culture characteristics: Colonies on PDA and OA at 25 °C reaching 2–4 and 4–10 mm diam respectively in 2 wk, velvety, brown-vinaceous, margin effuse; reverse dark; diffusible pigment saffron after 7 d. Sporulation abundant in the aerial and submerged mycelium.
Specimens examined: Spain, Aragón, Sierra y Cañones de Guara Natural Park, isolated from forest soil, Mar. 2011, M. Hernández-Restrepo & J. Capilla (holotype CBS H-22986; cultures ex-type CBS 142289, FMR 12149). Navarra, Valles Occidentales Natural Park, on submerged wood, Mar. 2012, M. Hernández-Restrepo & J. Capilla (CBS 142295, FMR 12418).
Notes: Cirrenalia comprises 13 species isolated from marine and terrestrial environments (Kohlmeyer 1966). Based on molecular and morphological data, several species were transferred to different genera, i.e. Halazoon, Hiogispora, Hydea and Matsusporium in the Lulworthiales (Abdel-Wahab et al. 2010).
Cirrenalia iberica is morphologically similar to C. macrocephala, C. pseudomacrocephala, C. basiminuta, and C. pallescens. However, it can be distinguished by its commonly straight conidia, in contrast to the coiled conidia of these latter species. In addition, the conidia of C. iberica are longer (19–41 μm) than those of C. macrocephala (12–35 μm) and C. pallescens (12.5–25 μm). The conidia of C. basiminuta are more septate (3–5 vs. 2–3 in C. iberica), and paler with a darker apical cell and a narrower basal cell (2.5–7 μm vs 3–9(–12.5) μm in C. iberica). Cirrenalia pseudomacrocephala has wider conidia (16–20 μm vs up to 15 μm wide in C. iberica) and with more septa (3–6).
Parasympodiellales Hern.-Restr., Gené, R.F. Castañeda & Crous, ord. nov. MycoBank MB820297.
Saprobic on leaves and twigs. Sexual morph. Unknown. Asexual morph. Conidiophores macronematous, mononematous, brown. Conidiogenous cells holoblastic, pale brown or hyaline. Conidia thallic-arthric, aseptate or septate, hyaline.
Type family: Parasympodiellaceae Hern.-Restr., Gené, Guarro & Crous.
Parasympodiellaceae Hern.-Restr., Gené, Guarro & Crous, fam. nov. MycoBank MB820298.
Conidiophores macronematous, mononematous, unbranched, brown. Conidiogenous cells holoblastic, sympodial, hyaline or pale brown, giving conidia in basipetal succession. Conidial secession schizolytic. Conidia thallic-arthric, aseptate or septate, cylindrical, hyaline, in unbranched, dry, basipetal, chains. Synasexual morph stylaspergillus-like often present. Conidiophores macronematous, mononematous, branched or unbranched, brown. Conidiogenous cells phialidic, formed on terminal or intercalary vesicle-like cells, pale brown. Conidia produced in slimy masses, filiform, hyaline.
Type genus: Parasympodiella Ponnappa.
Included genus: Parasympodiella.
Notes: Parasympodiellaceae and Parasympodiellales are introduced for the clade that encompasses four Parasympodiella species (Fig. 4, clade XVI), including P. laxa, the generic type, previously accommodated in Sympodiella (Ponnappa 1975). Sympodiella species have small conidiophores (up to 280 μm) with terminal or subterminal conidiogenous cells and conidial chains with up to six conidia (Kendrick 1958), while Parasympodiella has larger conidiophores (up to 700 μm), the conidiogenous cells are along the conidiophore stipe at irregular intervals and the conidia are produced in chains that appear to extend indefinitely. Furthermore, sequences of two strains of S. acicola (CBS 425.67 and CBS 487.82) include this genus in Venturiales (Dothideomycetes). Parasympodiella currently comprises 10 species, which are usually found colonising leaves and twigs of conifers and dicotyledonous plants (Crous et al., 1995, Cheewangkoon et al., 2009, Seifert et al., 2011).
Parasympodiella lauri Hern.-Restr., Gené & Guarro, sp. nov. MycoBank MB820299. Fig. 19.
Fig. 19.
Parasympodiella lauri (FMR 13132 ex-type). A. Conidiophores and conidiogenous cells. B, C. Conidia. Scale bars, A = 20 μm; B, C = 10 μm.
Etymology: The name refers to Laurus, the botanical host from which the species was found.
Colonies on the natural substratum effuse, like a white net. Mycelium mostly immersed, composed of brown, smooth hyphae. Conidiophores macronematous, mononematous, erect, unbranched, septate, cylindrical; sterile part with sligthly-thickened walls, brown, 100–300 × 6–8 μm; fertile part with thinner walls, pale brown, becoming paler toward the apex, 150–320 × 5–6 μm, with up to seven conidiogenous cells. Conidiogenous cells holothallic, terminal or intercalary, integrated, indeterminate, proliferating sympodially, smooth, pale brown, becoming hyaline toward the apex, 45–80 × 4–6 μm. Conidia thallic-arthric, forming unbranched, dry, chains, (0–)1-septate, cylindrical, (22–)27–40(–47) × 5–6(–7) μm, apex and base of intercalary conidia truncate, with a septal plug at each end, apical conidia with obtuse or rounded apex, hyaline, smooth, thin-walled. Stylaspergillus-like asexual morph not observed. Sexual morph not observed.
Culture characteristics: Colonies on PCA at 25 °C reaching 60 mm diam in 2 wk, with sparse aerial mycelium, zonate, dark green. Mycelium superficial or immersed, consisting of branched, septate, smooth, pale brown to dark brown hyphae, 2–12 μm wide. Conidia (0–)1(–2) septate, cylindrical or clavate 26–40(–50) × 5–9 μm. Chlamydospores present on the vegetative hyphae, intercalary, solitary or in short chains, spherical, 15–40 μm diam, brown, thin-walled, smooth, guttulate. Synasexual morph not observed.
Specimen examined: Spain, Canary Islands, La Palma, Biosphere Reserve Los Tilos, on fallen leaves of Laurus sp., Jul. 2013, M. Hernández-Restrepo & J. Guarro (holotype CBS H-21888; cultures ex-type FMR 13132, CBS 138108).
Notes: Parasympodiella lauri is morphologically similar to P. elongata and P. eucalypti in having cylindrical, (0–)1(–2) septate conidia. Nevertheless, on the natural substratum P. lauri has smaller conidia (22–47 × 5–7 μm) than those of P. elongata (30–65 × 6–8 μm), and P. eucalypti (25–65 × 8–11 μm) (Cheewangkoon et al. 2009). The phylogenetic tree (Fig. 4, Clade XVI) includes all the sequences of the different Parasympodiella species available for comparison, P. lauri being placed as sister to P. elongata.
Pleurotheciales, Pleurotheciaceae
Anapleurothecium Hern.-Restr., R.F. Castañeda & Gené, gen. nov. MycoBank MB820300.
Etymology: From the Greek, Ana-, meaning upwards, back, again; and -pleurothecium, referring to the asexual genus Pleurothecium. Morphologically similar, but distinct from Pleurothecium.
Colonies on the natural substratum effuse, hairy, dark brown to black. Mycelium mostly immersed, composed of septate, smooth, hyaline hyphae. Conidiophores macronematous, mononematous, unbranched, erect, straight, smooth, brown. Conidiogenous cells terminal or intercalary, polyblastic, sympodial, denticulate, brown. Conidial secession schizolytic. Conidia solitary, acropleurogenous, dry, septate, botuliform to cylindrical, rounded at both ends, smooth, brown, sometimes with a paler basal cell. Sexual morph unknown.
Type species: Anapleurothecium botulisporum Hern.-Restr., R.F. Castañeda & Gené.
Anapleurothecium botulisporum Hern.-Restr., R.F. Castañeda & Gené, sp. nov. MycoBank MB820301. Fig. 20.
Fig. 20.
Anapleurothecium botulisporum (FMR 11490 ex-type). A. Habit. B–E. Conidiogenous cells. F–L. Conidia. Scale bars = 10 μm.
Etymology: From the Latin botulus, which means “sausage”; and the Greek spore meaning “seed, sowing”. Named after the sausage-shape of its conidia.
Colonies on the natural substratum effuse, hairy, dark brown to black. Mycelium mostly immersed, composed of septate, smooth, hyaline hyphae. Conidiophores macronematous, mononematous, unbranched, erect, straight, cylindrical, 74–185 × 5–6 μm, smooth, brown. Conidiogenous cells terminal or intercalary, polyblastic, sympodial, denticulate, cylindrical, 10–47 × 4–7 μm, mid brown; denticles up to 4 μm long, 1 μm wide. Conidia solitary, (2–)3-septate, botuliform to cylindrical, 15–21 × 6–8.5 μm, smooth, brown, often with a basal cell pale brown.
Culture characteristics: Colonies on OA after 2 wk reaching up to 10 mm diam, flat, mycelium mainly submerged on the agar, with black spots corresponding to sporulating zones. Conidiophores and conidiogenous cells are similar to those observed on the natural substratum, producing slightly smaller conidia (15–20 × 5–7.5 μm).
Specimens examined: Spain, Asturias, Poncebos (Cares River), Picos de Europa National Park, on dead wood, Nov. 2010, M. Hernández-Restrepo, J. Guarro & J. Mena (holotype CBS H-20749; cultures ex-type FMR 11490, CBS 132713, IMI 502222, MUCL 54492); Cantabria, Saja-Besaya Natural Park, on dead wood, Nov. 2010, M. Hernández-Restrepo, J. Guarro & J. Mena (FMR 11580).
Notes: According to our phylogenetic analyses Anapleurothecium is placed in the Pleurotheciales (Fig. 4, Clade XVI; Fig. 5). This monotypic order was recently introduced with six clades (I–VI), and represents at least 11 genera (Réblová et al. 2016b). Anapleurothecium is related to “clade II” of Réblová et al. (2016b), that includes Helicoon farinosum, Monotosporella setosa (as Brachysporiella setosa), Phragmocephala stemphylioides and Pleurothecium obovoideum. These fungi are morphologically very different from Anapleurophragium as mentioned before. The most similar is P. obovoideum, but this differs in having shorter conidiophores (up to 35 μm long) and aseptate conidia, often arranged in short chains (Arzanlou et al. 2007). Anapleurothecium also resembles Pleurophragmium in having denticulate conidiogenous cells and more or less cylindrical conidia, but it mainly differs in having larger denticles (up to 4 μm) and darkly pigmented conidia. The conidiogenous cells in Pleurophragmium have shorter denticles (up to 2 μm long) and its conidia are hyaline or pale brown (Ellis, 1971, Seifert et al., 2011). In addition, the generic type, Pleurophragmium parvisporium, is related to the Papulosaceae (Réblová and Štěpánek, 2009, Jaklitsch et al., 2016a). Other genera with similar conidiogenous cells to Anapleurothecium are Camposporium and Paratrichoconis, but their conidial secession is rhexolytic, while in Anapleurothecium it is schizolytic. The phylogenetic position of Camposporium and Paratrichconis remains uncertain, and only species of the former genus have been related to Pleosporales (Fig. 1, clade III).
Savoryellales, Savoryellaceae
Neoascotaiwania Hern.-Restr., R.F. Castañeda & Guarro, gen. nov. MycoBank MB820302.
Etymology: Neo- meaning new; -ascotaiwania referring to the sexual genus Ascotaiwania. Name refers to the similarity with the genus Ascotaiwania.
Sexual morph. Ascomata perithecial, non-stromatic, semi-immersed, gradually erumpent to almost superficial, scattered or clustered in small groups, black, subglobose, papillate, with a periphysate ostiole. Ascomatal wall outer layer dark brown, textura prismatica, with pores; inner layer composed by hyaline, flattened cells. Asci cylindrical, with a non-amyloid, discoid apical ring, with 8 uniseriate ascospores. Paraphyses partially disintegrating at maturity, septate, branched, anastomosing. Ascospores ellipsoidal, septate, slightly constricted at the septa, versicolourous, middle cells brown, with small guttules, polar cells smaller and hyaline, smooth, without sheath or appendages. Asexual morph. Conidiophores micronematous, reduced to conidiogenous cells, hyaline to subhyaline. Conidiogenous cells monoblastic, integrated, arising directly from the hyphae. Conidial secession rhexolytic. Conidia solitary, dry, transversely septate, ellipsoidal to obovoid, dark brown, basal cell often paler, septa with darker bands.
Type species: Neoascotaiwania terrestris Hern.-Restr., R.F. Castañeda & Guarro.
Notes: Ascotaiwania was established with A. lignicola as type species, a fungus similar to Savoryella but distinguished by having asci with a prominent non-amyloid apical ring and 7-septate pigmented ascospores with hyaline end cells (Sivanesan & Chang 1992). Subsequently, some species with 3- or 5-septate ascospores were added to the genus, i.e. A. hsilio, A. sawada, A. palmicola, A. persoonii, A. hughesii, A. pallida and A. pennisetorum, as well as other species with 7-septate ascospores, i.e. A. wulai, A. mitriformis and A. mauritiana (Chang et al., 1998, Ranghoo and Hyde, 1998, Fallah et al., 1999, Hyde and Goh, 1999). Different asexual morphs have been observed for Ascotaiwania species: monodictys-like with multicellular dark brown conidia in A. lignicola (Chang 2001); trichocladium-like with 1-septate conidia, dark brown apical cell and hyaline and smaller basal cell in A. hsilio (Chang 2001); monotosporella-like in two species, i.e. A. sawada (Sivichai et al. 1998) and A. mitriformis (Ranghoo & Hyde 1998), and Helicoon farinosum for A. hughesii (Fallah et al. 1999). The current circumscription of Ascotaiwania is polyphyletic (Réblová et al. 2016b) (Fig. 5) and apparently like in other genera as Chaetosphaeria and Capronia, the asexual morph seems to be phylogenetically more relevant than the sexual morph. Neoascotaiwania differs from Ascotaiwania in having 3-septate ascospores, asci with thinner, non-amyloid apical ring, and the asexual morph is bactrodesmium-like.
Neoascotaiwania limnetica (H.S. Chang & S.Y. Hsieh) Hern.-Restr., R.F. Castañeda & Gené, comb. nov. MycoBank MB820303.
Basionym: Savoryella limnetica H.S. Chang & S.Y. Hsieh, Mycol. Res. 102: 715. 1998.
Synonym: Ascotaiwania limnetica (H.S. Chang & S.Y. Hsieh) Réblová & J. Fourn., Persoonia 37: 71. 2016.
Descriptions and illustrations: Chang et al., 1998, Réblová et al., 2016b.
Neoascotaiwania terrestris Hern.-Restr., R.F. Castañeda & Guarro, sp. nov. MycoBank MB820304. Fig. 21.
Fig. 21.
Neoascotaiwania terrestris (FMR 12412 ex-type). A–D. Conidiophores and conidia. E–J. Conidia. Scale bars = 10 μm.
Etymology: From the Latin –terra, meaning earth, soil, grown; since this fungus was isolated from a soil sample.
Description on OA. Mycelium partly immersed and partly superficial, composed of septate, smooth, hyaline to pale brown hyphae, 1.5–3 μm wide. Conidiophores micronematous, reduced to intercalary conidiogenous cells producing lateral blastic conidia. Conidia solitary, straight or curved, (2–)3–4(–5)-septate, ellipsoidal, obovoid, 25.5–44.5 × 13–22 μm, black to reddish brown, basal cell often subhyaline to pale brown or brown and truncate, 2.5–6 μm wide, smooth. Sexual morph not observed.
Culture characteristics: Colonies on PDA and OA at 25 °C reaching 4–5 mm diam in 2 wk, black, with aerial mycelium velvety, with a margin white and effuse; reverse black. Sporulation abundant in both submerged and superficial mycelium.
Specimen examined: Spain, Asturias, Picos de Europa National Park, isolated from forest soil, Oct. 2010, M. Hernández-Restrepo, J. Mena & J. Guarro (holotype CBS H-22988; cultures ex-type CBS 142291, FMR 12412).
Notes: Neoascotaiwania terrestris differs from N. limnetica in having larger conidia with a wider basal scar (25.5–44.5 × 13–22 μm, 2.5–6 μm vs 23–39 × 14.5–18.5 μm, base 3–4.5 μm in N. limetica) (Chang et al. 1998). Furthermore, N. limnetica is known from submerged dead wood in Taiwan and France (Chang et al., 1998, Réblová et al., 2016b), while N. terrestris was isolated from forest soil in Spain.
Sordariales, Chaetomiaceae
Trichocladium asperum (Corda) Harz. Fig. 22.
Basionym: Sporidesmium asperum Corda, Icon. fung. 2: 6. 1838.
Synonyms: Dicoccum asperum (Corda) Sacc., Syll. fung. 4: 342. 1886.
Monodictys aspera (Corda) S. Hughes, Canad. J. Bot. 36: 785. 1958.
Piricauda aspera (Corda) R.T. Moore, Rhodora 61: 96. 1959.
Specimens examined: Lectotype designated here: tab. VIII, fig. 27 in Corda ACJ, Icones Fungorum hucusque Cognitorum 2, 1838. MBT375510. Belgium, Kontich, isolated from agricultural soil, Jan. 1964, G.L. Hennebert (CBS 112.67). Germany, Edersee, Nieder-Werbe, isolated from acidic soil, E. Falk, No. C48, MBT375512 (epitype designated here CBS H-23060; culture ex-epitype CBS 903.85). Spain, Castilla La Mancha, Alto Tajo Natural park, isolated from soil, May 2011 M. Hernández-Restrepo, J. Mena & J. Guarro (FMR 12054). The Netherlands, unknown substrate, unknown date, C.M. Berkhout (CBS 140.21). Unknown country, unknown substrate, unknown date, O. da Fonseca (CBS 157.22).
Notes: Hughes (1952) lectotypified Trichocladium with T. asperum as type species. Unfortunately, the holotype of this species, formerly described as Sporidesmium (Corda 1838) seems to be lost. However, the protologue contains an illustration which is designated here as the lectotype of S asperum. We selected the strain CBS 903.85 as ex-epitype culture to fix the use of the name. CBS 903.85 matches with the protologue of the fungus described by Corda (1838) and it was collected in Germany, the same country where the species was formerly found. Our study agrees with Hambleton et al. (2005) confirming that T. asperum was phylogenetically related to Chaetomium and Humicola (Sordariales).
Vermiculariopsiellales Hern.-Restr., J. Mena, Gené & Crous, ord. nov. MycoBank MB820346.
Saprobic on leaves. Sexual morph. Unknown. Asexual morph. Conidiomata sporodochial, setose. Setae branched or unbranched. Conidiophores macronematous, densely packed in a palisade. Conidiogenous cells monophialidic, discrete. Conidia grouped in dry masses, unicellular or septate. Stroma present or absent.
Type family: Vermiculariopsiellaceae Hern.-Restr., J. Mena, Gené & Crous.
Vermiculariopsiellaceae Hern.-Restr., J. Mena, Gené & Crous, fam. nov. MycoBank MB820347.
Conidiomata sporodochial, scattered, setose. Setae branched or unbranched, brown. Conidiophores macronematous, densely packed in a palisade, pale brown or hyaline. Conidiogenous cells monophialidic, cylindrical to lageniform, with collarette, hyaline to pale brown. Conidia grouped in pale dry masses, unicellular or septate, cylindrical, hyaline. Stroma present or absent.
Type genus: Vermiculariopsiella Bender.
Included genus: Vermiculariopsiella.
Notes: In our phylogenetic analysis of the Sordariomycetes, the type species of Vermiculariopsiella, V. immersa, and other species of the genus, i.e. V. dichapetali, V. eucalypti, V. microsperma and V. pediculata, were placed in a monophyletic well-supported clade which is introduced in the present study as a new monotypic order and family (Fig. 4, clade XIII). Previous studies, based on culture techniques, reported Vermiculariopsiella spp. as asexual morphs of Echinosphaeria macrospora and E. pteridis (Gawas et al., 2006, Dhargalkar and Bhat, 2009); however, further molecular studies are needed to confirm this relationship, since the type species of Echinosphaeria, E. canescens, is related to the family Helminthosphaeriaceae in the order Chaetosphaeriales (Miller and Huhndorf, 2004, Miller et al., 2014).
Xenospadicoidales Hern.-Restr., J. Mena & Gené, ord. nov. MycoBank MB820348.
Saprobic on dead wood. Sexual morph. Unknown. Asexual morph. Conidiophores macronematous, mononematous, unbranched or branched. Conidiogenous cells tretic, integrated. Conidia solitary or in chains, dry, unicellular or septate.
Type family: Xenospadicoidaceae Hern.-Restr., J. Mena & Gené.
Xenospadicoidaceae Hern.-Restr., J. Mena & Gené, fam. nov. MycoBank MB820349.
Conidiophores macronematous, mononematous, erect, straight, unbranched or branched, brown. Conidiogenous cells tretic, terminal or intercalary, cylindrical, pale brown to brown. Conidia solitary or in chains, dry, unicellular or transversely septate, oblong, ellipsoidal or obovoid, brown.
Type genus: Xenospadicoides Hern.-Restr., J. Mena & Gené.
Included genera: Xenospadicoides, Pseudodiplococcium.
Xenospadicoides Hern.-Restr., J. Mena & Gené, gen. nov. MycoBank MB820350.
Etymology: Name reflects a morphological similarity with the genus Spadicoides.
Type species: Xenospadicoides atra (Corda) Hern.-Restr., J. Mena & Gené.
Colonies effuse, velvety, dark brown to black. Mycelium partly superficial, composed of branched, septate, pale to dark brown, smooth hyphae. Conidiophores single or in small groups, erect or ascending, straight or flexuous, unbranched, mid to dark brown paler toward the apex. Conidiogenous cells polytretic, integrated, terminal and intercalary, cylindrical. Conidia solitary, acropleurogenous, unicellular, ellipsoidal, oblong with rounded ends, occasionally obovoid, pale brown to very dark brown, smooth. Sexual morph unknown.
Xenospadicoides atra (Corda) Hern.-Restr., J. Mena & Gené, comb. nov. MycoBank MB820392.
Basionym: Psilonia atra Corda, Icon. fung. 4: 27. 1840.
Synonyms: Acladium atrum (Corda) Bonorden, Handbuch der allgemeinen Mycologie Stuttgart: 87. 1851.
Catenularia atra (Corda) Sacc., Syll. fung. 4: 304. 1886.
Spadicoides atra (Corda) S. Hughes, Canad. J. Bot. 36: 805. 1958.
Virgaria indivisa Sacc., Michelia 2: 560. 1882.
Diplococcium indivisium (Sacc.) Hughes, Canad. J. Bot. 31: 634. 1953.
Haplaria ellisii Cooke, Grevillea 17 (83): 69. 1889.
Trichosporium populneum Lambotte & Fautrey, Rev. Mycol. 18: 145. 1896.
Descriptions and illustrations: See Ellis, 1963, Hughes, 1973.
Specimens examined: Lectotype designated here: tab. VI, fig. 84 in Corda ACJ. Icones Fungorum hucusque Cognitorum 4, 1840, MBT376691. Czech Republic, Central Bohemia, forest Lánská obora, on branch of Quercus petraea, Jun. 1976, V. Holubová-Jechová No. 380, MBT376692 (epitype designated here CBS H-18296, culture ex-epitype CBS 489.77).
Notes: Because herbarium material of Psilonia atra, the basionym of Spadicoides atra, is not preserved and no authentic specimens have been located, we lectotypify the species with the original drawing included in the protologue of this fungus (Corda 1840). In addition, an ex-epitype culture (CBS 489.77) is designated here to fix the use of the name.
Xenospadicoides resembles Spadicoides in having polytretic conidiogenous cells that produce solitary conidia. However, these genera differ in their conidial septation, viz. 1-septate in Spadicoides and aseptate in Xenospadicoides. Furthermore, those two taxa are phylogenetically unrelated; Spadicoides bina is a member of the Cordanales (Shenoy et al., 2010, Hernández-Restrepo et al., 2014b, Hernández-Restrepo et al., 2015b) and Xenospadicoides forms a new lineage in Sordariomycetes (Fig. 4, clade X).
Pseudodiplococcium Hern.-Restr., J. Mena & Gené, gen. nov. MycoBank MB820353.
Etymology: Pseudo- meaning “false”; and diplococcium referring to the asexual genus Diplococcium. Morphologically similar to Diplococcium.
Colonies on the natural substratum effuse, velvety, brown to dark brown. Mycelium partly superficial, partly immersed in the substratum. Conidiophores macronematous, mononematous, extensively branched, dark brown, smooth. Conidiogenous cells polytretic, pores inconspicuous after conidial secession, integrated, terminal and intercalary, cylindrical, brown and smooth. Conidia in branched chains, dry, acropleurogenous, unicellular or septate transversely, cylindrical, pale brown to mid brown, concolourous, smooth.
Type species: Pseudodiplococcium ibericum Hern.-Restr., J. Mena & Gené.
Pseudodiplococcium ibericum Hern.-Restr., J. Mena & Gené, sp. nov. MycoBank MB820394.
Etymology: Refers to the name of the region, Iberian Peninsula, from where the species was collected.
Colonies on the natural substratum effuse, velvety, brown to dark brown. Mycelium partly superficial, partly immersed in the substratum. Conidiophores macronematous, mononematous, profusely branched, dark brown, smooth, up to 360 μm long, 3 μm wide, 3.5–5 μm at the apex, 5 μm at the base. Conidiogenous cells polytretic, pores inconspicuous after conidial secession, integrated, terminal and intercalary, cylindrical, brown and smooth. Conidia in branched chains, dry, acropleurogenous, (0–)1-septate, cylindrical, 7.5–8.5 × 4–4.2 μm (0-septate), 9–15.5(–17) × 4–5 μm (1-septate), pale brown to mid brown, concolourous, smooth.
Culture characteristics: Colonies on OA and PCA at 25 °C reaching 15 mm diam in 2 wk, cottony, convex and olive brown, margin regular to fimbriate; reverse sepia brown. Sporulation present after 2 wk. Conidia in long and often branched chains, (0–)1(–2)-septate, 6–9.5 × 4–5 μm (0-septate), 9–32 × 4–6 μm (1–2-septate).
Illustration: See Hernández-Restrepo et al. (2012).
Specimen examined: Spain, Valencia, Chera, Chera-Sot Natural Park, Pantano de Buseo, on dead wood, Mar. 2010, M. Hernández-Restrepo & K. Rodríguez (holotype CBS H-23059, cultures ex-type FMR 10959, CBS 127864).
Notes: The isolate FMR 10959 introduced here as P. ibericum, based on morphological data, was formerly identified as D. pulneyense (Hernández-Restrepo et al. 2012). This latter species is described as the asexual morph of Otthia pulneyensis (Subramanian & Sekar 1987), a presumed member of Dothideomycetes. Nevertheless, in our analysis, FMR 10959 is placed in a clade together with Xenospadicoides in Sordariomycetes (Fig. 4, clade X). After a re-examination of this material, we conclude that, in addition to the phylogenetic position, there are slight morphological differences which can help to distinguish both fungi. Although P. ibericum resembles D. pulneyense in having long chains of conidia born from polytretic conidiogenous cells, the former differs in the production of aseptate conidia occurring intercalary in the conidial chains, on both, culture and the natural substratum (Hernández-Restrepo et al. 2012). In D. pulneyense only septate conidia were described (Subramanian & Sekar 1987).
Xylariales
Castanediellaceae Hern.-Restr., Guarro & Crous, fam. nov. MycoBank MB820354.
Foliicolous, saprobic or associated to leaf spots. Sexual morph. Unknown. Asexual morph. Conidiophores macronematous, mononematous or aggregated in sporodochia, branched, brown to pale brown. Conidiogenous cells mono or polyblastic, sympodial, discrete, solitary or in whorls, cylindrical to lageniform, hyaline to subhyaline. Conidial secession schizolytic. Conidia unicellular or septate transversely, cylindrical, fusiform or lunate, hyaline.
Type genus: Castanediella Hern.-Restr., Crous & M.J. Wingf.
Included genus: Castanediella.
Notes: Castanediella was recently introduced with C. acacia as type species and six species are currently accepted (Crous et al., 2015b, Crous et al., 2016, Hernández-Restrepo et al., 2016). Castanediella as currently circumscribed is monophyletic (Fig. 4, clade I) and represents a distinct taxonomic group at the family level closely related to the Beltraniaceae in Xylariales.
Xylariales, Incertae sedis
Xyladictyochaeta Hern.-Restr., R.F. Castañeda & Gené, gen. nov. MycoBank MB820355.
Etymology: Xyla- referring to Xylariales; and -dictyochaeta referring to the asexual genus Dictyochaeta. Morphologically similar to Dictyochaeta but phylogenetically related to Xylariales.
Colonies on natural substratum effuse, hairy, brown. Mycelium partly superficial and partly immersed, composed of septate, hyaline or medium brown, smooth hyphae. Conidiophores macronematous, mononematous, setiform, commonly unbranched, rarely branched at the base, erect, cylindrical, brown, smooth. Conidiogenous cells integrated, mono- or polyphialidic and sympodial, collarette inconspicuous, terminal and usually lageniform, or intercalary and cylindrical with a lateral extension near a septum bearing terminally the conidiogenous loci, brown, smooth. Conidia solitary, mucous, 0–1-septate, falcate, base truncate, apex acute, hyaline, smooth, with an unbranched setula at each end.
Type species: Xyladictyochaeta lusitanica Hern.-Restr., R.F. Castañeda & Gené.
Notes: Morphologically, Xyladictyochaeta differs from Dictyochaeta in having setiform conidiophores with intercalary and terminal polyphialidic conidiogenous cells. In Dictyochaeta, the setae are mainly sterile and the conidiogenous cells are born from conidiophores in the lower part of the setae (Ellis, 1971, Réblová et al., 1999, Seifert et al., 2011). Moreover, several Dictyochaeta species are commonly associated as asexual morphs of Chaetosphaeria, a genus integrated in the Chaetosphaeriaceae (Chaetosphaeriales) (Réblová et al., 1999, Réblová, 2000, Réblová, 2004, Fernández and Huhndorf, 2005, Fernández et al., 2006).
Xyladictyochaeta lusitanica Hern.-Restr., R.F. Castañeda & Gené, sp. nov. MycoBank MB820356. Fig. 23.
Fig. 23.
Xyladictyochaeta lusitanica (FMR 12177 ex-type). A–D. Conidiophores. E. Conidia. Scale bars = 10 μm.
Description on OA. Mycelium composed of septate, hyaline or brown, smooth hyphae, 1–3 μm wide. Conidiophores macronematous, mononematous, erect, setiform, usually unbranched, some branched at the base, cylindrical, 40–110 × 3–5 μm, brown, smooth. Conidiogenous cells integrated, mono- or polyphialidic and sympodial, collarette inconspicuous, terminal and usually lageniform, 6–14.5 × 2.5–5 μm, or intercalary and cylindrical with a lateral extension near a septum, 2–5 × 1–2 μm. Conidia solitary, mucous, 0–1-septate, falcate, 11–16 × 2–2.5 μm, with a truncate base and an acute apex, hyaline, smooth, bearing an unbranched setula at each end, 3–7 μm long, up to 0.3 μm wide.
Culture characteristics: Colonies on OA and PDA at 25 °C reaching 24–25 mm diam in 2 wk, white and lanose, with white hyphal strands, submerged mycelium greenish grey in OA, umber in PDA, margin white, effuse; reverse greenish grey in OA, ochraceous in PDA. Sporulation abundant.
Specimen examined: Portugal, Viana do Castello, Lagoas de Bertiandos Protected Area, on dry leaves of Eucalyptus sp., Nov. 2011, M. Hernández-Restrepo, R.F. Castañeda & J. Gené (holotype CBS H-22986; cultures ex-type CBS 142290, FMR 12177).
Notes: Xyladictyochaeta lusitanica is similar to Dictyochaeta eucalypti; both species have setiform conidiophores with integrated, intercalary and terminal phialides and (0–)1-septate conidia with setulae at both ends (Sutton & Hodges 1975). Nevertheless, they differ by the morphology of their conidiogenous loci; X. lusitanica has polyphialides with denticles-like openings instead of monophialides with conspicuous flared to tubular collarettes as in D. eucalypti.
Sordariomycetes, Incertae sedis
Paradiplococcium Hern.-Restr., J. Mena & Gené, gen. nov. MycoBank MB820295.
Etymology: The name reflects the morphological similarity with Diplococcium.
Conidiophores macronematous, mononematous or in small fascicles, erect, straight, unbranched, brown. Conidiogenous cells polytretic, terminal and intercalary, integrated and cylindrical or discrete and globose, brown. Conidia in short dry, chains, acropleurogenous, septate, cylindrical, brown, smooth. Synasexual morph idriella-like in morphology is present in culture. Conidiophores macronematous, mononematous, bearing whorls of conidiogenous cells, or reduced to conidiogenous cells growing on Diplococcium conidia, pale brown, smooth. Conidiogenous cells polyblastic, discrete, sympodial, with a long and denticulate neck, lageniform, pale brown, smooth. Conidia often in slimy heads, unicellular, falcate or filiform, hyaline, smooth.
Type species: Paradiplococcium singulare (Hern.-Restr. et al.) Hern.-Restr., J. Mena & Gené.
Paradiplococcium singulare (Hern.-Restr. et al.) Hern.-Restr., J. Mena & Gené, comb. nov. MycoBank MB820296.
Basionym: Diplococcium singulare Hern.-Restr. et al., Mycol. Progr. 11: 194. 2012.
Description and illustration: See Hernández-Restrepo et al. (2012).
Specimen examined: Spain, Aragón, Ordesa y Monte Perdido National Park, Fanlo, Añisclo canyon, on dead wood, Jun. 2009, M. Hernández-Restrepo, J. Mena Portales & J. Cano (cultures ex-type CBS 126091, FMR 10752).
Notes: This species was formerly identified as Diplococcium because of the presence of polytretic and cylindrical conidiogenous cells that produce chains of septate and brown conidia (Hernández-Restrepo et al. 2012). However, the fungus also showed discrete and globose conidiogenous cells on the conidiophores, a feature not described in other species of Diplococcium. Therefore, the presence of these discrete conidiogenous cells and the production of short conidial chains allow us to distinguish Paradiplococcium from Diplococcium. In addition, the type species of this latter genus, D. spicatum, is related to Helotiales in Leotiomycetes (Shenoy et al. 2010).
Acknowledgements
The authors are grateful to Cony Decock (Université Catholique de Louvain, Belgium) and Kazuaki Tanaka (University of Hirosaki, Japan) for the valuable comments and critical review of this manuscript. Enrique Descals, Josep Cano, Javier Capilla and Kendra Rodriguez are thanked for their help during collection trips. We also thank the fungal collection at the Westerdijk Fungal Biodiversity Institute, the curator Gerard Verkley and the technical staff Trix Merkx, Yda Vlug and Arien van Iperen for providing the strains from the culture collection and for the deposit of herbarium material. This study was supported by the Spanish Ministerio de Economia y Competitividad, Grants CGL 2008-04226 and CGL 2011-27185.
Footnotes
Peer review under responsibility of Westerdijk Fungal Biodiversity Institute.
References
- Abdel-Wahab M.A., Pang K.L., Nagahama T. Phylogenetic evaluation of anamorphic species of Cirrenalia and Cumulospora with the description of eight new genera and four new species. Mycological Progress. 2010;9:537–558. [Google Scholar]
- Almeida D.A.C., Cruz A.C.R., Marques M.F.O. Conidial fungi from the semi-arid Caatinga biome of Brazil. New and interesting Zanclospora species. Mycosphere. 2013;4:684–692. [Google Scholar]
- Arzanlou M., Groenewald J.Z., Gams W. Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology. 2007;58:57–93. doi: 10.3114/sim.2007.58.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baral H.O. Nomenclatural novelties. Index Fungorum. 2015;271 [Google Scholar]
- Batista A.C. Dois novos gêneros de fungos imperfeitos. Boletim da Secretaria de Agricultura Indústria e Comércio do Estado de Pernambuco. 1952;19:107–111. [Google Scholar]
- Bender H.B. The genera of Fungi Imperfecti. Mycologia. 1932;24:410–412. [Google Scholar]
- Berkeley M.J., Broome C.E. Notices of British fungi (1038–1062) Annals and Magazine of Natural History. 1865;15:400–404. [Google Scholar]
- Blackwell M. The fungi: 1, 2, 3 … 5.1 million species? American Journal of Botany. 2011;98:426–438. doi: 10.3732/ajb.1000298. [DOI] [PubMed] [Google Scholar]
- Boonmee S., D'souza M.J., Luo Z. Dictyosporiaceae fam. nov. Fungal Diversity. 2016;80:457–482. [Google Scholar]
- Boonyuen N., Chuaseeharonnachai C., Suetrong S. Savoryellales (Hypocreomycetidae, Sordariomycetes): a novel lineage of aquatic ascomycetes inferred from multiple-gene phylogenies of the genera Ascotaiwania, Ascothailandia, and Savoryella. Mycologia. 2011;103:1351–1371. doi: 10.3852/11-102. [DOI] [PubMed] [Google Scholar]
- Cai L., Wu W.-P., Hyde K.D. Phylogenetic relationships of Chalara and allied species inferred from ribosomal DNA sequences. Mycological Progress. 2009;8:133. [Google Scholar]
- Calduch M., Gené J., Guarro J. Hyphomycetes from Nigerian rain forests. Mycologia. 2002;94:127–135. [PubMed] [Google Scholar]
- Calduch M., Gené J., Stchigel A. Ramophialophora, a new anamorphic genus of Sordariales. Studies in Mycology. 2004;50:83–88. [Google Scholar]
- Campbell J., Shearer C.A., Mitchell J.I. Corollospora revisited: a molecular approach. In: Hyde K.D., editor. Fungi in marine environments. Fungal Diversity Press; Hong Kong: 2002. pp. 15–33. [Google Scholar]
- Cano J., Guarro J., Gené J. Molecular and morphological identification of Colletotrichum species of clinical interest. Journal of Clinical Microbiology. 2004;42:2450–2454. doi: 10.1128/JCM.42.6.2450-2454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañeda-Ruiz R.F. Instituto de Investigaciones Fundamentales en Agricultura Tropical Alejandro de Humboldt; La Habana, Cuba: 1988. Fungi Cubenses III. [Google Scholar]
- Castañeda-Ruiz R.F., Guarro J., Cano J. Notes on conidial fungi. X. A new species of Ceratosporella and some new combinations. Mycotaxon. 1996;60:275–281. [Google Scholar]
- Castañeda-Ruiz R.F., Heredia G., Gusmao L.F.P. Fungal diversity of central and south America. In: De-Wei L., editor. Biology of microfungi. Springer International Publishing; Switzerland: 2016. pp. 197–218. [Google Scholar]
- Castañeda-Ruiz R.F., Hernández-Restrepo M., Gené J. Two new microfungi from Portugal: Magnohelicospora iberica gen. & sp. nov. and Phaeodactylium stadleri sp. nov. Mycotaxon. 2012;121:171–179. [Google Scholar]
- Castañeda-Ruiz R.F., Kendrick W.B. Conidial fungi from Cuba: I. University of Waterloo Biology Series. 1990;32:1–53. [Google Scholar]
- Chang H.S. Trichocladium anamorph of Ascotaiwania hsilio and Monodictys-like anamorphic states of Ascotaiwania lignicola. Fungal Science. 2001;16:35–38. [Google Scholar]
- Chang H.S., Hsieh S.Y., Jones E.B.G. New freshwater species of Ascotaiwania and Savoryella from Taiwan. Mycological Research. 1998;102:709–718. [Google Scholar]
- Cheewangkoon R., Groenewald J.Z., Hyde K.D. Chocolate spot disease of Eucalyptus. Mycological Progress. 2012;11:61–69. [Google Scholar]
- Cheewangkoon R., Groenewald J.Z., Summerell B.A. Myrtaceae, a cache of fungal biodiversity. Persoonia. 2009;23:55–85. doi: 10.3767/003158509X474752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda A.C.J. Vol. 1. 1837. (Icones Fungorum hucusque Cognitorum). i–iv, 1–32, plates 1–7. Prague. [Google Scholar]
- Corda A.C.J. Vol. 2. 1838. (Icones Fungorum hucusque Cognitorum). i–vi, 1–43, plates 1–15. Prague. [Google Scholar]
- Corda A.C.J. Vol. 4. 1840. (Icones Fungorum hucusque Cognitorum). i–iii, 1–53, plates 1–10. Prague. [Google Scholar]
- Crous P.W., Braun U., Groenewald J.Z. Mycosphaerella is polyphyletic. Studies in Mycology. 2007;58:1–32. doi: 10.3114/sim.2007.58.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Carris L.M., Giraldo A. The Genera of Fungi – fixing the application of the type species of generic names – G2: Allantophomopsis, Latorua, Macrodiplodiopsis, Macrohilum, Milospium, Protostegia, Pyricularia, Robillarda, Rotula, Septoriella, Torula, and Wojnowicia. IMA Fungus. 2015;6:163–198. doi: 10.5598/imafungus.2015.06.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Gams W., Stalpers J.A. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology. 2004;50:19–22. [Google Scholar]
- Crous P.W., Giraldo A., Hawksworth D.L. The Genera of Fungi: fixing the application of type species of generic names. IMA Fungus. 2014;5:141–160. doi: 10.5598/imafungus.2014.05.01.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Groenewald J.Z. Why everlastings don't last. Persoonia. 2011;26:70–84. doi: 10.3767/003158511X574532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Groenewald J.Z., Shivas R.G. Fungal Planet description sheets: 69–91. Persoonia. 2011;26:108–156. doi: 10.3767/003158511X581723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Schubert K., Braun U. Opportunistic, human-pathogenic species in the Herpotrichiellaceae are phenotypically similar to saprobic or phytopathogenic species in the Venturiaceae. Studies in Mycology. 2007;58:185–217. doi: 10.3114/sim.2007.58.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Shivas R.G., Quaedvlieg W. Fungal Planet description sheets: 214–280. Persoonia. 2014;32:184–306. doi: 10.3767/003158514X682395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Guarro J. Fungal Planet description sheets: 320–370. Persoonia. 2015;34:167–266. doi: 10.3767/003158515X688433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Kendrick W.B. Foliicolous dematiaceous hyphomycetes from Syzygium cordatum. Canadian Journal of Botany. 1995;73:224–234. [Google Scholar]
- Crous P.W., Wingfield M.J., Richardson D.M. Fungal Planet description sheets: 400–468. Persoonia. 2016;36:316–458. doi: 10.3767/003158516X692185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hoog G.S., Hermanides-Nijhof E.F. The black yeast and allied Hyphomycetes. Studies in Mycology. 1977;15:1–122. [Google Scholar]
- De Hoog G.S., Vicente V.A., Najafzadeh M.J. Waterborne Exophiala species causing disease in cold-blooded animals. Persoonia. 2011;27:46–72. doi: 10.3767/003158511X614258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhargalkar S., Bhat D.J. Echinosphaeria pteridis sp. nov. and its Vermiculariopsiella anamorph. Mycotaxon. 2009;108:115–122. [Google Scholar]
- Dudka I.A. New and rare species of fungi imperfecti from the basins of the southern part of Kiev Polessye. Ukrainskiy Botanichnyi Zhurnal. 1966;23:91–95. [Google Scholar]
- Egidi E., de Hoog G.S., Isola D. Phylogeny and taxonomy of meristematic rock-inhabiting black fungi in the Dothideomycetes based on multi-locus phylogenies. Fungal Diversity. 2014;65:127–165. [Google Scholar]
- Ellis M.B. Clasterosporium and some allied dematiaceae-phragmosporae. II. Mycological Papers. 1959;72:1–75. [Google Scholar]
- Ellis M.B. Dematiaceous hyphomycetes. V. Mycological Papers. 1963;93:1–33. [Google Scholar]
- Ellis M.B. Commonwealth Mycological Institute; Kew, UK: 1971. Dematiaceous hyphomycetes. [Google Scholar]
- Ertz D., Heuchert B., Braun U. Contribution to the phylogeny and taxonomy of the genus Taeniolella, with a focus on lichenicolous taxa. Fungal Biology. 2016;120:1416–1447. doi: 10.1016/j.funbio.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Fallah P.M., Crane J.L., Shearer C.A. Freshwater ascomycetes: two new species of Ascotaiwania from North America. Canadian Journal of Botany. 1999;77:87–92. [Google Scholar]
- Fernández F.A., Huhndorf S.M. New species of Chaetosphaeria, Melanopsammella and Tainosphaeria gen. nov. from the Americas. Fungal Diversity. 2005;18:15–57. [Google Scholar]
- Fernández F.A., Miller N.A., Huhndorf S.M. Systematics of the genus Chaetosphaeria and its allied genera: morphological and phylogenetic diversity in north temperate and neotropical taxa. Mycologia. 2006;98:121–130. doi: 10.3852/mycologia.98.1.121. [DOI] [PubMed] [Google Scholar]
- Fisher P.J., Webster J. The teleomorphs of Helicodendron giganteum and H. paradoxum. Transaction of the British Mycological Society. 1983;81:656–659. [Google Scholar]
- Gawas P., Shenoy B.D., Hyde K.D. Echinosphaeria macrospora sp. nov., teleomorph of Vermiculariopsiella endophytica sp. nov. Cryptogamie, Mycologie. 2006;27:11–20. [Google Scholar]
- Geiser D.M., Gueidan C., Miadlikowska J. Eurotiomycetes: Eurotiomycetidae and Chaetothyriomycetidae. Mycologia. 2006;98:1053–1064. doi: 10.3852/mycologia.98.6.1053. [DOI] [PubMed] [Google Scholar]
- Gönczöl J., Révay Á., Fisher P.J. Notes on Vargamyces aquaticus, a water borne dematiaceous hyphomycete. Mycotaxon. 1990;39:301–310. [Google Scholar]
- Hambleton S., Nickerson N.L., Seifert K.A. Leohumicola, a new genus of heat-resistant hyphomycetes. Studies in Mycology. 2005;53:29–52. [Google Scholar]
- Han J.G., Hosoya T., Sung G.H. Phylogenetic reassessment of Hyaloscyphaceae sensu lato (Helotiales, Leotiomycetes) based on multigene analyses. Fungal Biology. 2014;118:150–167. doi: 10.1016/j.funbio.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Harkness H.W. New species of Californian fungi. Bulletin of California Academy of Science. 1884;1:29–47. [Google Scholar]
- Hawksworth D.L. Fungal diversity and its implications for genetic resource collections. Studies in Mycology. 2004;50:9–18. [Google Scholar]
- Hawksworth D.L., Rossman A.Y. Where are all the undescribed fungi? Phytopathology. 1997;87:888–891. doi: 10.1094/PHYTO.1997.87.9.888. [DOI] [PubMed] [Google Scholar]
- Hernández-Restrepo M., Castañeda-Ruiz R.F., Gené J. Two new species of Solicorynespora from Spain. Mycological Progress. 2014;13:157–164. [Google Scholar]
- Hernández-Restrepo M., Gené J., Castañeda-Ruiz R. Emendation of the genus Bactrodesmiastrum (Sordariomycetes) and description of Bactrodesmiastrum monilioides sp. nov. from plant debris in Spain. Mycological Progress. 2015;14:1–7. [Google Scholar]
- Hernández-Restrepo M., Gené J., Mena-Portales J. New species of Cordana and epitypification of the genus. Mycologia. 2014;106:723–734. doi: 10.3852/13-122. [DOI] [PubMed] [Google Scholar]
- Hernández-Restrepo M., Groenewald J.Z., Crous P.W. Neocordana gen. nov., the causal organism of Cordana leaf spot on banana. Phytotaxa. 2015;205:229–238. [Google Scholar]
- Hernández-Restrepo M., Groenewald J.Z., Crous P.W. Taxonomic and phylogenetic re-evaluation of Microdochium, Monographella and Idriella. Persoonia. 2016;36:57–82. doi: 10.3767/003158516X688676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Restrepo M., Mena-Portales J., Gené J. New Bactrodesmiastrum and Bactrodesmium from decaying wood in Spain. Mycologia. 2013;105:172–180. doi: 10.3852/12-004. [DOI] [PubMed] [Google Scholar]
- Hernández-Restrepo M., Silvera-Simón C., Mena-Portales J. Three new species and a new record of Diplococcium from plant debris in Spain. Mycological Progress. 2012;11:191–199. [Google Scholar]
- Hibbett D.S., Binder M., Bischoff J.F. A higher-level phylogenetic classification of the Fungi. Mycological Research. 2007;111:509–547. doi: 10.1016/j.mycres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Holubová-Jechová V. Lignicolous Hyphomycetes from the Netherlands. Proceedings van de Koninklijke Nederlandse Akademie van Wetenschappen Section C. 1973;76:297–302. [Google Scholar]
- Hughes S.J. Studies on micro-fungi X. Zygosporium. Mycological Papers. 1951;44:1–18. [Google Scholar]
- Hughes S.J. Trichocladium Harz. Transactions of the British Mycological Society. 1952;35:152–157. [Google Scholar]
- Hughes S.J. Revisiones Hyphomycetum aliquot cum appendice de nominibus rejiciendis. Canadian Journal of Botany. 1958;36:727–836. [Google Scholar]
- Hughes S.J. New Zealand Fungi. 16. Brachydesmiella, Ceratosporella. New Zealand Journal of Botany. 1971;9:351–354. [Google Scholar]
- Hughes S.J. National Mycological Herbarium, Agriculture; Canada, Ottawa: 1973. Fungi Canadenses No 5. [Google Scholar]
- Hughes S.J. Heteroconium and Pirozynskiella n. gen., with comments on conidium trans-septation. Mycologia. 2007;99:628–638. doi: 10.3852/mycologia.99.4.628. [DOI] [PubMed] [Google Scholar]
- Hughes S.J., Kendrick W.B. New Zealand Fungi 4. Zanclospora gen. nov. New Zealand Journal of Botany. 1965;3:151–158. [Google Scholar]
- Huhndorf S.M., Miller A.N., Fernández F.A. Molecular systematics of the Sordariales: the order and the family Lasiosphaeriaceae redefined. Mycologia. 2004;96:368–387. [PubMed] [Google Scholar]
- Huhtinen S. Taxonomic studies in the genera Protounguicularia, Arachnopeziza and Dematioscypha. Mycotaxon. 1987;30:9–28. [Google Scholar]
- Huhtinen S. A monograph of Hyaloscypha and allied genera. Karstenia. 1989;29:45–252. [Google Scholar]
- Hyde K.D., Fröhlich J. Fungi from palms. XXXVII. The genus Astrosphaeriella, including ten new species. Sydowia. 1997;50:81–132. [Google Scholar]
- Hyde K.D., Goh T.K. Fungi on submerged wood from the River Coln, England. Mycological Research. 1999;103:1561–1574. [Google Scholar]
- Hyde K.D., Jones E.B.G., Lui J.-K. Families of Dothideomycetes. Fungal Diversity. 2013;63:1–313. [Google Scholar]
- Jaklitsch W.M., Baral H.-O., Lücking R. Ascomycota. In: Frey W., editor. Syllabus of plant families: Adolf Engler's Syllabus der Pflanzenfamilien. 1(2) Borntraeger Science Publishers; Stuttgart: 2016. pp. 1–322. [Google Scholar]
- Jaklitsch W.M., Gardiennet A., Voglmayr H. Resolution of morphology-based taxonomic delusions: Acrocordiella, Basiseptospora, Blogiascospora, Clypeosphaeria, Hymenopleella, Lepteutypa, Pseudapiospora, Requienella, Seiridium and Strickeria. Persoonia. 2016;37:82–105. doi: 10.3767/003158516X690475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Voglmayr H. Hidden diversity in Thyridaria and a new circumscription of the Thyridariaceae. Studies in Mycology. 2016;85:35–64. doi: 10.1016/j.simyco.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James T.Y., Kauff F., Schoch C. Reconstructing the early evolution of the fungi using a six gene phylogeny. Nature. 2006;443:818–822. doi: 10.1038/nature05110. [DOI] [PubMed] [Google Scholar]
- Johnston P.R. The Bloxamia anamorph of Bisporella discedens. Mycotaxon. 1988;31:345–350. [Google Scholar]
- Johnston P.R., Seifert K.A., Stone J.K. Recommendations on generic names competing for use in Leotiomycetes (Ascomycota) IMA Fungus. 2014;5:91–120. doi: 10.5598/imafungus.2014.05.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten P.A. Fragmenta mycologica XXXIX. Hedwigia. 1892;31:297–299. [Google Scholar]
- Katoh, Standley MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick W.B. Sympodiella a new hyphomycetes genus. Transaction of the British Mycological Society. 1958;41:519–521. [Google Scholar]
- Kendrick W.B., Bhatt G.C. Trichocladium opacum. Canadian Journal of Botany. 1966;44:1728–1730. [Google Scholar]
- Kohlmeyer J. Neue Meerespilze an Mangroven. Berichte der Deutschen Botanischen Gesellschaft. 1966;79:27–37. [Google Scholar]
- Korf R.P., Carpenter S.E. Bisporella, a generic name for Helotium citrinum and its allies, and the generic names Calycella and Calycina. Mycotaxon. 1974;1:51–62. [Google Scholar]
- Koukol O., Kolárová Z. Bactrodesmium gabretae (anamorphic Helotiales), a new sporodochial species described from spruce needles. Nova Hedwigia. 2010;91:243–248. [Google Scholar]
- Linder D.H. A monograph of the helicosporous Fungi Imperfecti. Annals of the Missouri Botanical Garden. 1929;16:227–348. [Google Scholar]
- Liu J.K., Phookamsak R., Jones E.B.G. Astrosphaeriella is polyphyletic with species in Fissuroma gen. nov., and Neoastrosphaeriella gen. nov. Fungal Diversity. 2011;51:135–154. [Google Scholar]
- Liu X.Z., Wang Q.M., Göker M. Towards an integrated phylogenetic classification of the Tremellomycetes. Studies in Mycology. 2015;81:85–147. doi: 10.1016/j.simyco.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.Z., Wang Q.M., Theelen B. Phylogeny of tremellomycetous yeast and related dimorphic and filamentous basidiomycetes reconstructed from multiple gene sequence analyses. Studies in Mycology. 2015;81:1–26. doi: 10.1016/j.simyco.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locquin M. Masson; Paris: 1984. Mycologie générale et structurale. [Google Scholar]
- Lombard L., Houbraken J., Decock C. Generic hyper-diversity in Stachybotriaceae. Persoonia. 2016;36:156–246. doi: 10.3767/003158516X691582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Van der Merwe N.A., Groenewald J.Z. Generic concepts in Nectriaceae. Studies in Mycology. 2015;80:189–245. doi: 10.1016/j.simyco.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Zhuang W.Y. Chaetopsinectria (Nectriaceae, Hypocreales), a new genus with Chaetopsina anamorphs. Mycologia. 2010;102:976–984. doi: 10.3852/09-263. [DOI] [PubMed] [Google Scholar]
- Madrid H., Hernández-Restrepo M., Gené J. New and interesting chaetothyrialean fungi from Spain. Mycological Progress. 2016;15:1179–1201. [Google Scholar]
- Magyar D., Révay A. New species of Oncopodiella (Hyphomycetes) from living trees. Nova Hedwigia. 2009;88:169–182. [Google Scholar]
- Mantle P.G., Hawksworth D.L., Pazoutova S. Amorosia littoralis gen. sp. nov., a new genus and species name for the scorpinone and caffeine-producing hyphomycete from the littoral zone in The Bahamas. Mycological Research. 2006;110:1371–1378. doi: 10.1016/j.mycres.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Mason E.W. Annotated account of fungi received at the Imperial Mycological Institute. Mycological Papers. 1941;5:103–144. [Google Scholar]
- Mason E.W., Hughes S.J. Phragmocephala gen. nov. Hyphomycetarum. The Naturalist London. 1951;1951:97–105. [Google Scholar]
- Matsushima T. Matsushima Mycological Memoirs. 1983;3:1–90. [Google Scholar]
- Matsushima T. Matsushima Mycological Memoirs. 1993;7:1–141. [Google Scholar]
- Matsushima T. Matsushima Mycological Memoirs. 1996;9:1–30. [Google Scholar]
- McLaughlin D.J., Hibbett D.S., Lutzoni F. The search for the fungal tree of life. Trends in Microbiology. 2009;17:488–497. doi: 10.1016/j.tim.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Mel'nik V.A., Shabunin D.A. Rare or rarely revealed fungi Monodictys capensis and Peyronelina glomerulata. Mikologiya i Fitopatologiya. 2010;44:410–413. [Google Scholar]
- Mena-Portales J., Guarro J., Gené J. Taxonomy, distribution and conservation status of some interesting hyphomycetes (anamorphic fungi) from La Palma Biosphere Reserve, Canary Islands. Boletin de la Sociedad Micologica de Madrid. 2015;39:15–28. [Google Scholar]
- Mena-Portales J., Hernández-Restrepo M., Guarro J. New species of Penzigomyces, Sporidesmium and Stanjehughesia from Spain. Nova Hedwigia. 2016;103:359–371. [Google Scholar]
- Mena-Portales J., Hernández-Restrepo M., Minter D.W. A new species of Ceratocladium from Spain. Mycological Progress. 2011;10:493–496. [Google Scholar]
- Miller A.N., Huhndorf S.M. A natural classification of Lasiosphaeria based on nuclear LSU rDNA sequences. Mycological Research. 2004;108:26–34. doi: 10.1017/s0953756203008864. [DOI] [PubMed] [Google Scholar]
- Miller A.N., Huhndorf S.M., Fournier J. Phylogenetic relationships of five uncommon species of Lasiosphaeria and three new species in the Helminthosphaeriaceae (Sordariomycetes) Mycologia. 2014;106:505–524. doi: 10.3852/13-223. [DOI] [PubMed] [Google Scholar]
- Miller M.A., Pfeiffer W., Schwartz T. Proceedings of the Gateway Computing Environments Workshop (GCE), November 14, 2010, New Orleans, Louisiana. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic tree; pp. 1–8. [Google Scholar]
- Minter D.W., Watson A.T. Acrospermum chilense and Acrospermales. Boletin de la Sociedad Argentina de Botanica. 2007;42:107–112. [Google Scholar]
- Müller E., Petrini O., Fisher P.J. Taxonomy and anamorphs of the Herpotrichiellaceae with notes on generic synonymy. Transactions of the British Mycological Society. 1987;88:63–74. [Google Scholar]
- Nag-Raj T.R., Kendrick W.B. Wilfrid Laurier University Press; Waterloo, Ontario, Canada: 1975. Monograph of Chalara and allied genera. [Google Scholar]
- Nannizzi A. Repertorio sistematico dei miceti dell' uomo e degli animali. Trattato di Micopatologia Umana. 1934;4:1–557. [Google Scholar]
- O'Donnell K. Fusarium and its near relatives. In: Reynolds R., Taylor J.W., editors. The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CBA International; Wallingford, UK: 1993. pp. 225–233. [Google Scholar]
- Pfister P. Castor, Pollux and life histories of fungi. Mycologia. 1997;89:1–23. [Google Scholar]
- Ponnappa K.M. Parasympodiella gen. nov. Transactions of the British Mycological Society. 1975;64:344–345. [Google Scholar]
- Quaedvlieg W., Binder M., Groenewald J.Z. Introducing the consolidated species concept to resolve species in the Teratosphaeriaceae. Persoonia. 2014;33:1–40. doi: 10.3767/003158514X681981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W., Groenewald J.Z., de Jesús Yáñez-Morales M. DNA barcoding of Mycosphaerella species of quarantine importance to Europe. Persoonia. 2012;29:101–115. doi: 10.3767/003158512X661282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quijada L., Huhtinen S., Negrín R. Studies in Hyaloscyphaceae associated with major vegetation types in the Canary Islands II: a revision of Hyaloscypha. Willdenowia. 2017;47:31–42. [Google Scholar]
- Ranghoo V.M., Hyde K.D. Ascomycetes from freshwater habitats: Ascolacicola aquatica gen. et sp. nov. and a new species of Ascotaiwania from wood submerged in a reservoir in Hong Kong. Mycologia. 1998;90:1055–1062. [Google Scholar]
- Rayner R.W. British Mycological Society. Commonwealth Mycological Institute; Kew, Surrey: 1970. A mycological colour chart. [Google Scholar]
- Réblová M. The genus Chaetosphaeria and its anamorphs. Studies in Mycology. 2000;45:149–168. [Google Scholar]
- Réblová M. Four new species of Chaetosphaeria from New Zealand and re-description of Dictyochaeta fuegiana. Studies in Mycology. 2004;50:171–186. [Google Scholar]
- Réblová M., Barr M.E., Samuels G.J. Chaetosphaeriaceae, a new family for Chaetosphaeria and its relatives. Sydowia. 1999;51:49–70. [Google Scholar]
- Réblová M., Miller A.N., Rossman A.Y. Recommendations for competing sexual-asexually typified generic names in Sordariomycetes (except Diaporthales, Hypocreales, and Magnaporthales) IMA Fungus. 2016;7:131–153. doi: 10.5598/imafungus.2016.07.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réblová M., Seifert K.A. Discovery of the teleomorph of the hyphomycete, Sterigmatobotrys macrocarpa, and epitypification of the genus to holomorphic status. Studies in Mycology. 2011;68:193–202. doi: 10.3114/sim.2011.68.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réblová M., Seifert K.A., Fournier J. Phylogenetic classification of Pleurothecium and Pleurotheciella gen. nov. and its dactylaria-like anamorph (Sordariomycetes) based on nuclear ribosomal and protein-coding genes. Mycologia. 2012;104:1299–1314. doi: 10.3852/12-035. [DOI] [PubMed] [Google Scholar]
- Réblová M., Seifert K.A., Fournier J. Newly recognised lineages of perithecial ascomycetes: the new orders Conioscyphales and Pleurotheciales. Persoonia. 2016;37:57–81. doi: 10.3767/003158516X689819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réblová M., Štěpánek V. New fungal genera, Tectonidula gen. nov. for Calosphaeria-like fungi with holoblastic-denticulate conidiogenesis and Natantiella gen. nov. for three species segregated from Ceratostomella. Mycological Research. 2009;113:991–1002. doi: 10.1016/j.mycres.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Réblová M., Untereiner W.A., Réblová K. Novel evolutionary lineages revealed in the Chaetothyriales (Fungi) based on multigene phylogenetic analyses and comparison of its secondary structure. PLoS One. 2013;8:e63547. doi: 10.1371/journal.pone.0063547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Révay A., Gönczöl J., Merényi Z. Re-examination of Vargamyces aquaticus – a dematiaceous hyphomycete species. Sydowia. 2014;66:69–78. [Google Scholar]
- Rifai M.A. On Sporidesmium trigonellum Sacc. Persoonia. 1965;3:407–411. [Google Scholar]
- Ronquist F., Teslenko M., van der Mark P. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccardo P.A. Fungi Gallici lecti a Cl. viris P. Brunaud, C.C. Gillet, Abb. Letendre, A. Malbranche, J. Therry & Dom. Libert. Series IV. Michelia. 1882;2:583–648. [Google Scholar]
- Samuels G.J. Ascomycetes of New Zealand 1. Ohleria brasiliensis new record and its Monodictys anamorph with notes on taxonomy and systematics of Ohleria and Monodictys. New Zealand Journal of Botany. 1980;18:515–523. [Google Scholar]
- Schoch C.L., Crous P.W., Groenewald J.Z. A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology. 2009;64:1–15. doi: 10.3114/sim.2009.64.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch C.L., Seifert K.A., Huhndorf S. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert K., Morgan-Jones G., Gams W., Kendrick B. Vol. 9. Westerdijk Fungal Biodiversity Institute; Utrecht, The Netherlands: 2011. The genera of Hyphomycetes. (CBS Biodiversity series). [Google Scholar]
- Selbmann L., de Hoog G.S., Mazzaglia A. Fungi at the edge of life: cryptoendolithic black fungi from Antarctic desert. Studies in Mycology. 2005;51:1–32. [Google Scholar]
- Shearer C.A., Raja H.A., Miller A.N. The molecular phylogeny of freshwater Dothideomycetes. Studies in Mycology. 2009;64:145–153. doi: 10.3114/sim.2009.64.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy B.D., Jeewon R., Wang H. Sequence data reveals phylogenetic affinities of fungal anamorphs Bahusutrabeeja, Diplococcium, Natarajania, Paliphora, Polyschema, Rattania and Spadicoides. Fungal Diversity. 2010;44:161–169. [Google Scholar]
- Sinclair R.C., Boshoff S., Eicker A. A new species of Monodictys from South Africa. Mycotaxon. 1996;59:359–366. [Google Scholar]
- Sivanesan A., Chang H.S. Ascotaiwania, a new amphisphaeriaceous genus on wood from Taiwan. Mycological Research. 1992;96:481–484. [Google Scholar]
- Sivichai S., HyweI-Jones N., Jones E.B.G. Lignicolous freshwater Ascomycota from Thailand: 1. Ascotaiwania sawada and its anamorph state Monotosporella. Mycoscience. 1998;39:307–311. [Google Scholar]
- Somrithipol S., Jones E.B.G. Lauriomyces cylindricus and Lauriomyces ellipticus spp. nov., two new hyphomycetes from tropical forest of Thailand. Nova Hedwigia. 2007;84:479–486. [Google Scholar]
- Spatafora J.W., Johnson D., Sung G.-H. A five-gene phylogenetic analysis of the Pezizomycotina. Mycologia. 2006;98:1018–1028. doi: 10.3852/mycologia.98.6.1018. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014 doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stielow J.B., Lévesque C.A., Seifert K.A. One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia. 2015;35:242–263. doi: 10.3767/003158515X689135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Udayanga D., Luo Z. Hyphomycetes from aquatic habitats in Southern China: Species of Curvularia (Pleosporaceae) and Phragmocephala (Melannomataceae) Phytotaxa. 2015;226:201–216. [Google Scholar]
- Subramanian G.V., Sekar G. Three bitunicate ascomycetes and their tretic anamorphs. Kavaka. 1987;15:87–97. [Google Scholar]
- Sutton B.C. Hyphomycetes from Manitoba and Saskatchewan, Canada. Mycological Papers. 1973;132:1–143. [Google Scholar]
- Sutton B.C. Notes on some deuteromycetes genera with cheiroid or digitate brown conidia. Proceedings of the Indian Academy of Science. 1985;94:229–244. [Google Scholar]
- Sutton B.C., Hodges C.S. Eucalyptus microfungi: Codinaea and Zanclospora species from Brazil. Nova Hedwigia. 1975;26:517–525. [Google Scholar]
- Tamura K., Stecher G., Peterson D. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Hirayama K., Yonezawa H. Revision of the Massarineae (Pleosporales, Dothideomycetes) Studies in Mycology. 2015;82:75–136. doi: 10.1016/j.simyco.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui C.K.M., Berbee M.L. Phylogenetic relationships and convergence of helicosporus fungi inferred from ribosomal DNA sequences. Molecular Phylogenetics and Evolution. 2006;39:587–597. doi: 10.1016/j.ympev.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Tsui C.K.M., Berbee M.L. Transfer of two Helicoma species to Troposporella based on molecular and morphological data. Mycoscience. 2010;51:144–148. [Google Scholar]
- Untereiner W.A., Straus N.A., Malloch D. A molecular-morphotaxonomic approach to the systematics of the Herpotrichiellaceae and allied black yeasts. Mycological Research. 1995;99:897–913. [Google Scholar]
- Vu D., Groenewald M., Szöke S. DNA barcoding analysis of more than 9000 yeast isolates contribute to quantitative thresholds for yeast species and genera delimitation. Studies in Mycology. 2016;85:95–105. doi: 10.1016/j.simyco.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.M., Begerow D., Groenewald Multigene phylogeny and taxonomic revision of yeast and related fungi in the Ustilaginomycotina. Studies in Mycology. 2015;81:55–83. doi: 10.1016/j.simyco.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.M., Groenewald M., Takashima M. Phylogeny of yeasts and related filamentous fungi within Pucciniomycotina determined from multigene sequences analysis. Studies in Mycology. 2015;81:27–53. doi: 10.1016/j.simyco.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.M., Yurkov A.M., Göker M. Phylogenetic classification of yeast and related taxa within Pucciniomycotina. Studies in Mycology. 2015;81:149–189. doi: 10.1016/j.simyco.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Houbraken J., Groenewald J.Z. Diversity and taxonomy of Chaetomium and chaetomium-like fungi from indoor environments. Studies in Mycology. 2016;84:145–224. doi: 10.1016/j.simyco.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Lombard L., Groenewald J.Z. Phylogenetic reassessment of the Chaetomium globosum species complex. Persoonia. 2016;36:83–133. doi: 10.3767/003158516X689657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Binder M., Schoch C.L. Evolution of helotialean fungi (Leotiomycetes, Pezizomycotina): a nuclear rDNA phylogeny. Molecular Phylogenetetics and Evolution. 2006;41:295–312. doi: 10.1016/j.ympev.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Webster J. Conidia of Acrospermum compressum and A. graminum. Transaction of the British Mycological Society. 1956;39:361–366. [Google Scholar]
- White T.J., Bruns T., Lee S. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR protocols: a guide to methods and applications. Academic Press; New York, USA: 1990. pp. 315–322. [Google Scholar]
- Wu H., Jaklitsch W.M., Voglmayr H. Epitypification, morphology, and phylogeny of Tothia fuscella. Mycotaxon. 2011;118:203–211. [Google Scholar]
- Wu W.P., Zhuang W.Y. Fungal Diversity Press; Thailand: 2005. Sporidesmium, Endophragmiella and related genera from China. (Fungal Diversity Research Series 15). [Google Scholar]
- Yang J., Maharachchikumbura S.S.N., Bhat D.J. Fuscosporellales, a new order of aquatic and terrestrial Hypocreomycetidae(Sordariomycetes) Cryptogamie, Mycologie. 2016;37:449–475. [Google Scholar]
- Zhang Y., Crous P.W., Schoch C.L. A molecular, morphological and ecological re-appraisal of Venturiales a new order of Dothideomycetes. Fungal Diversity. 2011;51:249–277. doi: 10.1007/s13225-011-0141-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Crous P.W., Schoch C.L. Pleosporales. Fungal Diversity. 2012;53:1–221. doi: 10.1007/s13225-011-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Fournier J., Crous P.W. Phylogenetic and morphological assessment of two new species of Amniculicola and their allies (Pleosporales) Persoonia. 2009;23:48–54. doi: 10.3767/003158509X472187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Schoch C.L., Fournier J. Multi-locus phylogeny of the Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Studies in Mycology. 2009;64:85–102. doi: 10.3114/sim.2009.64.04. [DOI] [PMC free article] [PubMed] [Google Scholar]