Abstract
Objectives
Our study sought to compare the overall survival in patients with hepatocellular carcinoma (HCC) and portal venous thrombosis (PVT), treated with either conventional trans-arterial chemoembolization (cTACE) or drug-eluting beads (DEB) TACE.
Methods
This retrospective analysis included a total of 133 patients, treated without cross-over and compared head-to-head by means or propensity score weighting. Mortality was compared using survival analysis upon propensity score weighting. Adverse events and liver toxicity grade ≥3 were recorded and reported for each TACE. In order to compare with historical sorafenib studies, a sub-group analysis was performed and included patients who fulfilled the SHARP inclusion criteria.
Results
The median overall survival (MOS) of the entire cohort was 4.53 months (95 % CI, 3.63–6.03). MOS was similar across treatment arms, no significant difference between cTACE (N =95) and DEB-TACE (N =38) was observed (MOS of 5.0 vs. 3.33 months, respectively; p = 0.157). The most common adverse events after cTACE and DEB- TACE, respectively, were as follows: post-embolization syndrome [N =57 (30.0 %) and N =38 (61.3 %)], diarrhea [N =3 (1.6 %) and N =3 (4.8 %)], and encephalopathy [N =11 (5.8 %) and N=2 (3.2 %)].
Conclusion
Our retrospective study could not reveal a difference in toxicity and efficiency between cTACE and DEB-TACE for treatment of advanced stage HCC with PVT.
Keywords: Liver, Hepatocellular carcinoma, Chemoembolization, Adverse effects, Propensity score
Introduction
Hepatocellular carcinoma (HCC) is the second most common cause of cancer-related death in the world, with higher incidences in Asian countries and rising incidences in Europe and the United States [1–3]. HCC is a locally highly invasive cancer and is often diagnosed at intermediate and advanced stages (Barcelona Clinic Liver Cancer [BCLC] stage B or C) [4]. According to the European Association for the Study of the Liver (EASL) guidelines, catheter-based intra-arterial therapies (IAT) and systemic chemotherapy with sorafenib are the recommended standard of care for patients with BCLC stage B and C, respectively [1, 5, 6]. The dual benefit of IAT lies in their ability to deliver a high concentration of chemotherapeutic agents or local radiation directly to the tumor, while reducing the systemic toxicity of the delivered payload [5, 7].
According to the BCLC staging system, one of the contraindications for trans-arterial chemoembolization (TACE) is portal-venous thrombosis (PVT). The reported incidence of macrovascular tumor invasion in general and PVT in particular is as high as 37 % in patients with HCC [8–10]. While not officially endorsed by the BCLC staging system, evidence exists in support of IAT in this subset of patients. There have been several reports that demonstrated the safety and efficacy of IAT in patients with PVT and therefore, IATs are widely used in this subset of patients around the world [11–14]. Contrary to the BCLC recommendations, the recently introduced Hong Kong Liver Cancer (HKLC) staging system proposed a more aggressive therapeutic algorithm by recommending IAT in patients with intrahepatic vascular invasion and differentiate between intra- and extrahepatic vascular invasion as opposed to BCLC [8, 15]. Yet, there is no consensus regarding the choice between conventional TACE (cTACE) and drug-eluting beads TACE (DEB-TACE) in this subset of patients, and until today, no study has answered this question [7, 16].
Our study sought to compare the overall survival in patients with HCC and PVT, treated with either conventional TACE or DEB-TACE without therapy cross-over (head-to-head comparison) by means of propensity score weighting using the BCLC staging system parameters.
Materials and methods
Study cohort
This retrospective single-institution study was conducted in compliance with the Health Insurance Portability and Accountability Act and approved by the institutional review board (IRB). Between 2000 and 2013, a total of 813 patients with HCC were treated with IAT. The criteria for inclusion and exclusion of patients are itemized within the flowchart (Fig. 1).
Fig. 1.
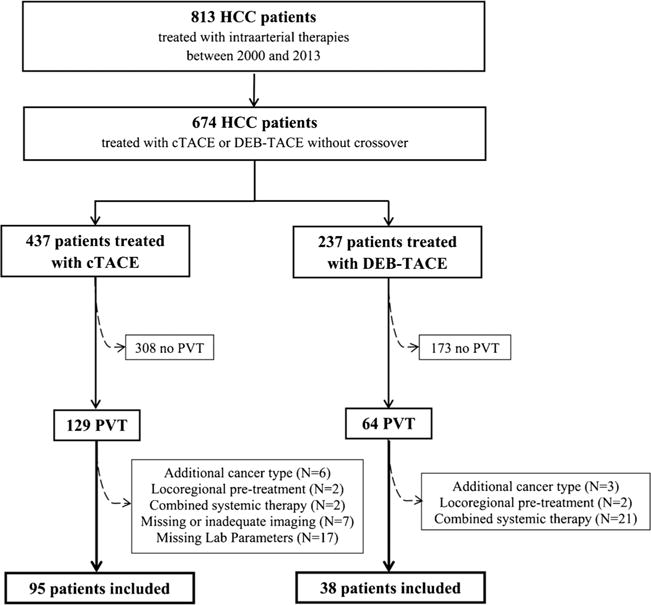
Inclusion criteria flowchart. PVT, portal venous thrombosis; cTACE, conventional transarterial chemoembolization; DEB-TACE, drug-eluting beads TACE
Our study included patients who received TACE between 2000 and 2013. Therefore, a substantial portion of the analyzed data stems from an era before the introduction of DEB-TACE in 2006 [17] and sorafenib (SHARP-trial, 2008) [9]. Consequently, between 2000 and 2008 patients were predominantly treated with cTACE, and between 2009 and 2013 with DEB-TACE. It was only more recently that both therapies were used parallel in this institution. There has been no clear institutional decision algorithm in the choice of cTACE vs. DEB-TACE. Moreover, according to the already published literature (Table 1), no clear superiority of Yttrium-90 Radioembolization (Y90 RE), sorafenib or TACE could be demonstrated. Therefore, the choice of treatment has been decided case-by-case within the inter-disciplinary tumor board (consisting of interventional radiologists, medical oncologists and liver surgeons), and by a discussion with the patient himself.
Table 1.
Selected summary of the survival outcome from patients with HCC and PVT after TACE, sorafenib or Y90 RE
| Studies | Group | N | Treatment | OS in months (95 % CI) | p Value |
|---|---|---|---|---|---|
| Sorafenib | |||||
| 2009 Yau [18] | PVT | 22 | Sorafenib | 5 | |
| 2012 Cheng [19] | PVT and/or extrahepatic metastasis | 118 | Sorafenib | 5.6 (4.8–6.7) | |
| 2012 Bruix (Sub-analyses from the SHARP trial) [20] | PVT | 108 | Sorafenib | 8.1 | |
| 123 | Placebo | 4.9 | |||
| Sorafenib vs. Y90 RE | |||||
| 2015 Edeline [21] | PVT | 34 | glass Y90 RE | 18.3 (12.0–28.9) | <0.001 |
| 117 | Sorafenib | 6.5 (5.4–8.1) | |||
| 2016 de la Torre [22] | PVT | 26 | resin Y90 RE | 8.8 (1.8–15.8) | 0.047 |
| 47 | Sorafenib | 5.4 (2.7–8.1) | |||
| Y90 RE | |||||
| 2008 Kulik [23] | peripheral PVT | 25 | glass Y90 RE | 10.1 (7.2–16.0) | |
| main PVT | 12 | glass Y90 RE | 4.5 (2.9–7.5) | ||
| 2010 Salem [24] | PVT + Child Pugh A | 35 | glass Y90 RE | 10.4 (7.2–16.6) | |
| PVT + Child Pugh B | 57 | glass Y90 RE | 5.6 (4.5–6.7) | ||
| 2010 Hilgard [25] | PVT | 33 | glass Y90 RE | 10.0 (6.0–n.a.) | |
| 2013 Memon [26] | peripheral PVT (only Child Pugh A) | 19 | glass Y90 RE | 15.7 (9.0–20.0) | |
| main PVT (only Child Pugh A) | 16 | glass Y90 RE | 9.0 (5.0–14.0) | ||
| 2016 Biederman [27] | PVT | 69 | glass Y90 RE | 9.5 (7.6–15.0) | <0.001 |
| 21 | resin Y90 RE | 3.7 (2.3–6.0) | |||
| Y90 RE vs. TACE | |||||
| 2010 Carr [28] | PVT | 295 | cTACE | 7 (5–9) | |
| PVT | 28 | glass Y90 RE | 5 (4–9) | ||
| TACE vs. BSC | |||||
| 1997 Lee [29] | main PVT | 31 | cTACE | 5.0 | 0.11 |
| 16 | BSC | 3.0 | |||
| 2011 Chung [14] | main PVT | 83 | cTACE | 5.6 | <0.001 |
| 42 | BSC | 2.2 | |||
| 2011 Luo [30] | peripheral PVT | 40 | cTACE | 10.2 | 0.002 |
| 24 | BSC | 5.2 | |||
| main PVT | 44 | cTACE | 5.3 | 0.002 | |
| 56 | BSC | 3.4 | |||
| 2012 Niu [31] | PVT | 115 | cTACE | 8.67 | <0.001 |
| 35 | BSC | 1.4 | |||
| TACE | |||||
| 1995 Chung [32] | PVT | 110 | cTACE | 6.0 | |
| 2005 Georgiades [13] | PVT | 32 | cTACE | 9.5 (3–50) | |
| 2012 Kalva [11] | peripheral PVT | 10 | DEB-TACE | 11.4 (0–22.7) | |
| 2013 Prajapati [12] | PVT | 41 | DEB-TACE | 8.8 (3.9–13.6) | |
| 2014 Liu [33] | main PVT | 90 | cTACE | 4.1 | <0.0001 |
| peripheral PVT | 98 | cTACE | 8.4 | ||
| 2014 Zhu [34] | main PVT | 10 | cTACE + Sorafenib | 3.0 | 0.588 |
| 11 | cTACE | 3.0 | |||
| First order PVT | 19 | cTACE + Sorafenib | 13.0 | 0.002 | |
| 21 | cTACE | 6.9 | |||
| Second or lower order PVT | 17 | cTACE + Sorafenib | 15.0 | 0.003 | |
| 13 | cTACE | 10.0 | |||
| 2016 Huang [35] | PVT | 140 | cTACE | 7.5 | |
| 2016 Li [36] | PVT | 735 | cTACE | 4.8 (4.4–5.2) | |
cTACE, conventional trans-arterial chemoembolization; DEB-TACE, drug-eluting beads TACE; BSC, best supportive care; Y90 RE, Yttrium 90 radioembolization; MOS, median overall survival; CI, confidence interval; HCC, hepatocellular carcinoma; PVT, portal venous thrombosis;
Our study included 36 patients treated with cTACE prior to clinical introduction of DEB-TACE in 2006. The cTACE group was divided into two groups, before and after 2006. Accordingly, three analyses were conducted: 1) the entire cTACE cohort (2000–2013) vs. the DEB-TACE cohort; 2) patients treated with cTACE (200–2013) upon introduction of DEB-TACE were compared with the DEB-TACE cohort; and 3) the cTACE cohort (2000–2006) was compared with the other cTACE cohort (2006–2013).
Overall, 194 cTACE procedures (mean 2.0, range 1–10) and 63 DEB-TACE procedures (mean 1.7, range 1–4) were performed. Table 2 shows the baseline patient characteristics. In order to allow for a direct comparison of our data with survival outcomes reported in the setting of systemic chemotherapy (most notably within the Sorafenib HCC Assessment Randomized Protocol [SHARP] trial [9], as well as the Asia-Pacific trial [19]), a sub-group analysis was performed and included patients who fulfilled the SHARP inclusion criteria (BCLC C, Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 2 and Child-Pugh class A; [SHARP criteria]). After excluding all patients with Child-Pugh > A (58 and 20 patients from the cTACE and DEB-TACE group, respectively), no one from the remaining patients had an ECOG PS > 2 or had a BCLC D, and therefore, no one was further excluded from this sub-analysis. A total of 37 and 18 patients (cTACE and DEB-TACE, respectively) were included in the analysis according to the SHARP inclusion criteria.
Table 2.
Baseline patient characteristics—Pre-weighting
| Parameter | N | N |
|---|---|---|
| Treatment | cTACE | DEB-TACE |
| Demographics | 95 | 38 |
| Age | ||
| >65 years | 28 (27.4 %) | 16 (42.1 %) |
| ≤65 years | 67 (72.6 %) | 22 (57.9 %) |
| Sex | ||
| Male | 85 (89.5 %) | 30 (78.9 %) |
| Female | 10 (10.5 %) | 8 (21.1 %) |
| Cirrhosis | ||
| Absent | 9 (9.5 %) | 6 (15.8 %) |
| Present | 86 (90.5 %) | 32 (84.2 %) |
| Staging System | ||
| BCLC class | ||
| C | 77 (81.1 %) | 32 (84.2 %) |
| D | 18 (18.9 %) | 6 (15.8 %) |
| ECOG PS score | ||
| 0 | 29 (31 %) | 6 (15.8 %) |
| 1 | 58 (61 %) | 25 (65.8 %) |
| 2 | 3 (3 %) | 4 (10.5 %) |
| 3 | 5 (5 %) | 3 (7.9 %) |
| Child Pugh class | ||
| A | 37 (39 %) | 18 (47.4 %) |
| B | 44 (46 %) | 17 (44.7 %) |
| C | 14 (15 %) | 3 (7.9 %) |
| Tumor characteristics | ||
| Portal Venous Thrombosis | ||
| peripheral | 22 (23.2 %) | 13 (34.2 %) |
| main | 33 (34.7 %) | 12 (31.6 %) |
| extrahepatic | 40 (42.1 %) | 13 (34.2 %) |
| Multiplicity | ||
| unifocal | 8 (8.4 %) | 8 (21.1 %) |
| multifocal | 87 (91.6 %) | 30 (78.9 % |
| Size of the dominant lesion (D | iameter) | |
| BCLC | ||
| ≤3 cm | 4 (4.2 %) | 2 (5.3 %) |
| >3 cm | 91 (95.8 %) | 36 (94.7 %) |
| Tumor burden | ||
| ≤50 % | 63 (66.3 %) | 27 (71.1 %) |
| >50 % | 32 (33.7 %) | 11 (28.9 %) |
| Tumor type | ||
| nodular | 35 (36.8 %) | 22 (57.9 %) |
| infiltrative | 60 (63.2 %) | 16 (42.1 %) |
| Lobe | ||
| left | 1 (1.1 %) | 3 (8 %) |
| right | 10 (10.5 %) | 8 (21 %) |
| bilobar | 84 (88.4 %) | 27 (71 %) |
| Extrahepatic Metastasis | ||
| no | 86 (90.5 %) | 35 (92.1 %) |
| yes | 9 (9.5 %) | 3 (7.9 %) |
| Lymph Node Metastasis* | ||
| no | 40 (42.1 %) | 12 (31.6 %) |
| yes | 55 (57.9 %) | 26 (68.4 %) |
Enlargement of intrahepatic and paraortic lymph nodes on contrast enhanced magnetic resonance imaging or computed tomography
cTACE, conventional trans-arterial chemoembolization; DEB-TACE, drug-eluting beads TACE; BCLC, Barcelona Clinic Liver Cancer; ECOG PS, Eastern Cooperative Oncology Group performance status
Overall survival
The values reported in the time-to-event analysis (i.e., overall survival) refer to the date of the first TACE session as the study entry point and the date of death (N =99) as the endpoint. Patients who were lost to follow-up (N = 21) or changed the treatment method (such as liver transplantation [N =5] or sorafenib [N = 8]) were censored.
Treatment history, toxicity report
All clinical and laboratory adverse events were reported per TACE-procedure according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.03. The mean post-procedural hospital stay was 1.2 and 1.4 days for cTACE and DEB-TACE, respectively, and ranged between one and 15 days and one and eight days for cTACE and DEB-TACE, respectively.
Treatment
All TACE procedures were performed by one experienced interventional radiologist (JFG with currently 19 years of experience in hepatic interventions). A consistent approach according to our IRB-approved institutional protocols was used in all patients. First, multiple angiographic steps were performed to define the hepatic arterial anatomy, to determine portal venous patency and to evaluate tumor vascularity. Angiography was performed from the superior mesenteric artery, celiac axis as well as selectively in the right or left hepatic artery. Injection rates (2–5 mL/sec for a duration of 2–4 seconds) varied according to the blood vessel caliber and flow (Medrad, Warrendale, Pennsylvania, USA). The contrast agent used was Oxilan (Guerbet, France). For cTACE, patients were treated selectively (lobar or segmental) and super-selectively (subsegmental). An oil-in-water emulsion was created by mixing 10 ml Lipiodol (Guerbet, France) in a 1:1 ratio with 10 ml 0.9 % saline solution containing the drug cocktail of dissolved 50 mg doxorubicin and 10 mg mitomycin-C, and followed by administration of gelfoam, 300–500 or 100–300 μm diameter microspheres (Embospheres, Merit Medical, South Jordan, Utah, USA). Substantial arterial flow reduction to the tumor was defined as the technical endpoint of embolization by measuring the time it takes to clear the contrast column (typically 2–5 heart beats). For DEB-TACE, patients were treated selectively with 100–300 μm LC Beads (Biocompatibles/BTG, Surrey, United Kingdom). Up to 4 ml of DEBs (loaded with 25 to 37.5 mg of doxorubicin per milliliter of beads) were administered by alternating injections of aliquots of the beads and non-ionic contrast, with a total maximum dose of up to 100 mg doxorubicin delivered to the targeted tissue. Complete stasis was avoided to maintain arterial patency in order to allow re-treatment. Re-treatment was performed with the initial treatment modality if there was no response or disease progression on contrast-enhanced magnetic resonance imaging (MRI). No rigid embolization schedule was used.
Imaging technique
122 patients underwent a standardized liver MRI protocol including breath-hold unenhanced and contrast-enhanced imaging before the initial TACE. Eleven patients received multidetector computed tomography (CT) on baseline imaging. CT Images were acquired using a standard abdominal scan protocol with acquisitions before and after intra-venous administration of iodine-based contrast.
Imaging data evaluation
Two radiological readers who did not perform the TACE (RS and JC with 10 and 2 years of experience with abdominal MRI, respectively) performed the assessment of all baseline and follow-up images. Any ambiguities were resolved by consensus. PVT was defined either by arterial hyper-enhancement and venous or delayed-phase washout or by restricted diffusion within the portal vein on contrast-enhanced MR or CT images that were acquired no earlier than one month prior to the first TACE session [37]. The localization of the vascular invasion was classified as either main PVT if the main portal vein or the confluence of the left and right portal vein was affected, or peripheral PVT if the first and/or second order of the portal vein was involved [34]. Patients with an isolated hepatic invasion were not considered as PVT and were excluded.
Statistical analysis
Propensity score adjustment
The study design and statistical analysis of the data was performed by two senior statisticians (E.A.S. and B.A.S.N.) with extensive experience with propensity score matching techniques. A propensity score approach was used to generate a data set that is balanced in the observed covariates (Supplementary Table 1) across the two treatment-regimens (cTACE and DEB-TACE). Weighting was chosen because it allowed us to use all individuals available in our relatively small sample. The propensity score adjustment is further described in the supplementary section.
Time-to-event analyses
Kaplan-Meier survival curves were plotted and we fitted a propensity score-weighted Cox proportional hazards regression model for time to death. All statistical analyses were conducted in R 3.0.3 (R Core Team [2014]). The propensity score weighting was done using the add-in R package twang and the survival analysis using the package survival [38].
Results
After propensity score weighting, a good balance was achieved in the covariates defined by the BCLC staging system; the absolute standardized difference in means for all of the included covariates was less than 0.1 and it was below the recognized threshold value of balance of 0.2 (Supplementary Table 2).
Study Cohort sub-analysis before vs. after the introduction of DEB-TACE in 2006
The unadjusted survival comparison for patients treated with cTACE before and after 2006 showed no significant difference (median overall survival [MOS] was 4.5 months [95 % CI, 3.7–9.7] before and 5.0 months [95 % CI, 2.7–8.1] after 2006 [p = 0.56]). Comparable results were observed after comparing only patients who were treated after 2006. The survival was 5.00 months (95 % CI, 2.8–8.1) vs. 3.33 months (95 % CI, 2.7–6.2) for cTACE and DEB-TACE, respectively (p = 0.153). After adjusting for the covariates used in the propensity score weighting (Supplementary Table 1), treatment remained non-significant (after 2006 vs. before 2006 [p = 0.97; HR, 1.0]). Similar results were observed after comparing only patients who were treated after 2006. After adjusting for the above-mentioned covariates, treatment type remained non-significant (DEB-TACE vs. cTACE [p = 0.34; HR, 1.31]).
Survival analysis
Survival analysis was performed to compare outcomes between the two treatment arms. The median overall survival (MOS) of the entire cohort was 4.53 months (95 % CI, 3.63–6.03). Most importantly, the comparison of patient survival according to treatment modality showed no significant difference after propensity score weighting (5.00 [95 % CI, 4.03–6.07] vs. 3.33 [95 % CI, 3.00–5.33] months, p = 0.157, Fig. 2). MOS of patients with BCLC C and D without propensity score weighting was 5.00 months (95 % CI, 3.97–6.23) and 1.50 months (95 % CI, 1.23–n/a), respectively (p = 0.00142).
Fig. 2.
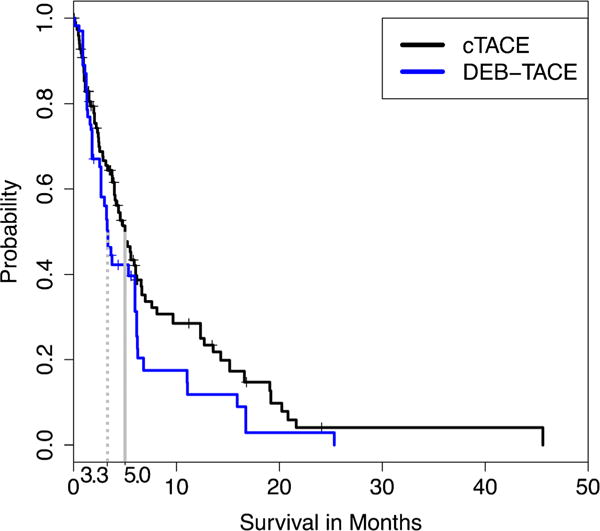
Kaplan-Meier curves demonstrating survival after propensity score weighting. The propensity score model has used the covariates defined by BCLC. Survival was defined as the time from the date of TACE to the date of death from any cause. Patients who were lost to follow-up or received another therapy (such as liver transplantation or sorafenib) were censored. BCLC, Barcelona Clinic Liver Cancer; cTACE, conventional transarterial chemoembolization; DEB-TACE, drug-eluting beads TACE
The subgroup survival analysis according to the SHARP criteria demonstrated a MOS of 8.1 months vs. 5.3 months in the cTACE and DEB-TACE group, respectively (p = 0.053) (Fig. 3).
Fig. 3.
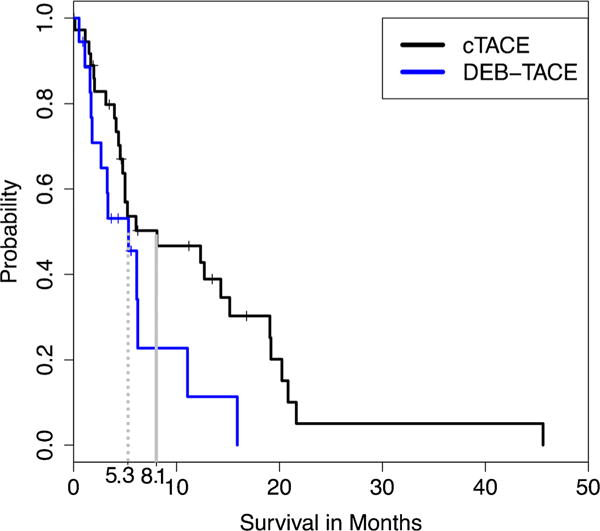
Survival comparison of cTACE and DEB-TACE according to the SHARP inclusion criteria [9]. Survival sub-analysis with characteristics from the SHARP trial [9] and the Asia-Pacific trial [19] (BCLC C, ECOG PS ≤ 2 and Child-Pugh class A; [SHARP criteria]). SHARP, Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol; cTACE, conventional trans-arterial chemoembolization; DEB-TACE, drug-eluting beads TACE
Multivariate Cox proportional hazards regression
Treatment modality (DEB-TACE or cTACE) was found to have no significant effect on survival before adjusting for other potential confounding covariates. This was true even after adjusting for the covariates included in the propensity score weighting (DEB-TACE vs. cTACE [p = 0.11; HR, 1.43]). It was found that after propensity score weighted analyses, Child-Pugh class C vs. A+B (p =0.013; HR, 3.07) and tumor burden>50 % (p = 0.0001; HR, 3.02) were the only independent predictive factors for patient survival (Table 3).
Table 3.
Cox hazard regression analysis after propensity score weighting
| Hazard Ratio | p value | |
|---|---|---|
| Intraarterial Therapy (DEB-TACE vs. cTACE) | 1.43 | 0.11 |
| PVT (main vs. peripheral) | 0.86 | 0.57 |
| Child-Pugh (C vs. A–B) | 3.07 | 0.013 |
| Cirrhosis (yes vs. no) | 1.11 | 0.73 |
| ECOG PS score (0 vs. 1–2 or ≥3) | 0.39 | 0.096 |
| ECOG PS score (1–2 vs. 0 or ≥3) | 0.47 | 0.17 |
| Multiplicity (unifocal vs. multifocal) | 1.6 | 0.21 |
| Size of the dominant lesion (>3 cm vs. ≤ 3 cm) | 0.53 | 0.16 |
| Tumor burden (>50 % vs. ≤ 50 %) | 3.02 | 0.0001 |
| Tumor type (infiltrative vs. nodular) | 1.15 | 0.58 |
| Lobe (unilobar vs. bilobar) | 0.694 | 0.23 |
| Extrahepatic Metastasis (yes vs. no) | 1.092 | 0.85 |
cTACE, conventional trans-arterial chemoembolization; DEB-TACE, drug-eluting beads TACE; PVT, portal venous thrombosis; ECOG PS, Eastern Cooperative Oncology Group performance status
Toxicity report
The most common adverse events after cTACE and DEB-TACE, respectively, were as follows: post-embolization syndrome (fatigue, nausea, vomiting, abdominal pain, fever without infection focus) [N =57 (30.0 %) and N =38 (61.3 %)], diarrhea [N =3 (1.6 %) and N =3 (4.8 %)], encephalopathy [N =11 (5.8 %) and N =2 (3.2 %)] (Table 4). A complete list of all occurred adverse events and a separation into 0–7 and 8–30 days are listed in Supplementary Table 3.
Table 4.
Summary from the most common Clinical Adverse Events and Laboratory Toxicities grade 3 or higher per procedure according to CTCAEv4.03
| cTACE | DEB-TACE | p value | |
|---|---|---|---|
| Clinical Toxicity | N =190 | N = 62 | |
| PES | 57 (30.0 %) | 38 (61.3 %) | 0.63 |
| diarrhea | 3 (1.6 %) | 3 (4.8 %) | 0.67 |
| encephalopathy | 11 (5.8 %) | 2 (3.2 %) | 0.83 |
| 30-Day-Mortality | 11 (5.7 %) | 4 (6.3 %) | |
| Biochemical Toxicity | N =107 | N =47 | |
| Albumin | 5 (4.7 %) | 0 (0.0 %) | n/a |
| Bilirubin | 22 (20.6 %) | 5 (10.6 %) | 0.78 |
| ALP | 7 (6.5 %) | 5 (10.6 %) | 0.85 |
| ALT | 12 (11.2 %) | 5 (10.6 %) | 0.98 |
| AST | 20 (18.7 %) | 11 (23.4 %) | 0.92 |
| Ammonia | 20 (18.7 %) | 9 (19.1 %) | 0.99 |
The toxicity report refers to all occurred adverse events within 30 days per procedure from all available post-embolic reports (N =252) or available laboratory data (154), respectively. Laboratory events refer to grade 3 or higher except of ammonia. Latter describes an increased level. Death refers to all procedures and is not limited to the availability of the post-procedural clinical reports
CTCAEv4.03; common terminology criteria for adverse events version 4.03; cTACE, conventional trans-arterial chemoembolization; DEB-TACE, drug-eluting beads TACE; BCLC, Barcelona Clinic Liver Cancer; PES, post embolic syndrome (fatigue, nausea, vomiting, abdominal pain, fever without infection focus); ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; n/a, not applicable
Discussion
Our study did not identify substantial treatment modality-related differences in MOS among patients with HCC and PVT who received cTACE or DEB-TACE. However, a subgroup analysis in cTACE patients who fulfilled the SHARP trial inclusion criteria revealed a MOS of 8.1 months, thus matching the life expectancy of comparable patients treated with sorafenib [9, 20].
Several published studies identified PVT as a negative prognostic factor in patients with HCC, which is also reflected in the design of both BCLC and the HKLC staging systems [4, 8]. The bulk of published data includes patients with mostly peripheral, segmental intrahepatic PVT and largely well preserved liver function (Child-Pugh A) [26, 39]. At the same time, very few studies included large numbers of patients with main PVT who underwent IAT, and, for the most part, had poor survival outcomes (Table 1) [14, 23].
The clear clinical evidence for the safety of IATs in such patient cohorts, however, has not yet been translated into reliable recommendations for the choice of treatment modality [11–14, 23]. Currently, Y90 RE is favored by some authors in patients with PVT [23–25]. In a scenario with absent or minimal portal-venous blood flow and arterially supplied healthy liver tissue, both clinically available radioembolization devices (TheraSpheres® and SIRSpheres®) are widely considered as less micro-embolic as compared with the larger embolic particles or microspheres used for TACE [40, 41]. However, this theoretically lower risk of liver infarction and post embolic syndrome (PES) for radioembolization has not yet been confirmed in a dedicated prospective trial and did not translate into clinical benefits with regard to patient survival [28]. As such and in light of the herein presented results, TACE remains an equally safe and effective treatment modality vis-à-vis patients with HCC and PVT.
Our propensity score weighted analysis failed to show a survival benefit for patients with PVT who received DEB-TACE (MOS = 3.33 months) over those treated with cTACE (MOS = 5.00 months, p = 0.157). These results are inline with the published literature for early to intermediate stage, which showed no significant difference between DEB-TACE and cTACE in term of survival, yet demonstrated a better toxicity profile in the DEB-TACE arm [16, 42, 43]. Evidence exists that DEB-TACE is also safe in advanced stage disease [11, 12] and as presented in this study, equally safe to cTACE in patients with PVT. Due to the missing survival benefit and the missing beneficial toxicity profile of the more expensive treatment modality DEB-TACE over cTACE, we see no rationality in using DEB-TACE in this subset of patient. Therefore, we will prefer cTACE over DEB-TACE in patients with HCC and PVT.
In our cohort of advanced through end-stage HCC patients, the BCLC staging system recommends systemic chemotherapy with sorafenib or best supportive care as the only treatment option [10]. However, there is clear evidence for potential survival benefits in patients with HCC irrespective of the stage when treated with IAT as compared to those who received best supportive care [29, 44]. Specifically, a subgroup analysis in cTACE patients who fulfilled the SHARP trial inclusion criteria revealed a MOS of 8.1 months (with a shorter MOS of 5.3 months for the DEB-TACE group), thus matching the life expectancy of comparable patients treated with sorafenib within the SHARP trial [9, 20]. Even though the shorter MOS of DEB-TACE is not significant, we suggest that this might be explained with the unique characteristics of Lipiodol, which functions both as an embolic agent as well as a drug carrier with the ability to deliver the chemotherapeutic component of the payload deep inside of the tumor and through arterioportal communication such as the peribiliary vascular plexus into the portal vein and thus potentially within the portal-venous thrombus [14, 39, 45–48]. As for DEB-TACE, our protocol utilized beads with diameters of 100–300 μm that are known for their ability to deliver the drug selectively to the tumor while reducing systemic toxicity [7]. However, these microsphere carriers may not be able to penetrate their target deep enough and beyond the arterioles in order to exhibit sufficient anti-tumoral effects within the PVT.
Moreover, the improved survival of the subgroup analysis according to the SHARP trial inclusion criteria compared to the entire group can be explained by the exclusion of 78 patients with Child-Pugh > A. Latter in combination with a tumor burden >50 % have proved to be the only independent predictive factor for survival in the present study. These results indicate a truly competitive role of cTACE in this subpopulation of patients, while surpassing the patient outcome reported in a similar subgroup in the Asia-Pacific trial (MOS 5.6 months) [19]. This observation is in line with the already published data in which sorafenib failed to show a survival benefit over cTACE in BCLC C and over Y90 RE in PVT (Table 1) [21, 22, 49]. In light of the relatively high overall incidence of minor (Grade I–II, 71.9–84.9 %) and severe adverse events (Grade III–IV, 52–54 %) in patients who were continuously treated with sorafenib [9, 20], TACE appears as a safer and effective alternative. In our study cohort, PES was the most frequently observed toxicity and occurred in 37.6 % of the BCLC C patients. Aspartate transaminase elevation as the most commonly observed laboratory toxicity (Grade III–IV) in the BCLC C group was less frequent in TACE as compared with similar, sorafenib-treated BCLC C populations (17.2 % vs. 41 %) [18]. In contrast to that, some published works show that sorafenib might not be tolerated well in patients with reduced liver function, and may even result in extremely poor survival outcomes (MOS in Child-Pugh A 8.9 months vs. 2 months in Child-Pugh B, p = 0.04) and an unfavorable toxicity profile (liver dysfunction in 21 % vs. 35 %, fatigue in 58 % vs. 82 % in Child-Pugh A and Child-Pugh B, respectively) [50]. It can thus be concluded that TACE may very well offer equal or better survival benefits in this subgroup of patients while showing a better toxicity profile in patients with PVT and reduced liver function.
There are some limitations to our study. First, our analysis was based on a retrospective cohort. Therefore, selection bias and confounders cannot be fully excluded. However, propensity score weighting makes group similar with respect to observed characteristics and limits the unadjusted confounders to the unobserved ones [51]. Second, the present study is characterized by a very long recruiting time, which may invariably skew the data due to adjustments of TACE protocols and overall technical innovations. Third, our patient selection criteria led to the exclusion of a significant subset of patients with PVT who were treated with DEB-TACE in combination with sorafenib (in the framework of a Phase II trial, NCT00844883). This exclusion criterion was justified with the fact that potential effects of sorafenib on patient survival cannot be compensated within the analysis because no cTACE patient received sorafenib; i.e., the DEB-TACE and cTACE groups could not be balanced with respect to this factor. Accordingly, our cohort of DEB-TACE patients was potentially less representative as compared to the selection of patients treated with cTACE. Fourth, there has been no clear institutional decision algorithm in the choice of cTACE vs. DEB-TACE, and therefore, the choice of treatment has been decided case-by-case within the inter-disciplinary tumor board and by a discussion with the patient himself.
Our retrospective study could not reveal a difference in toxicity and efficiency between cTACE and DEB-TACE for treatment of advance staged HCC with PVT. Further, our subgroup analysis suggests that IAT with TACE can be seen as an alternative for systemic therapy with sorafenib in selected patient cohorts with PVT. Overall, only prospective randomized trials that would include a head-to-head comparison between TACE and sorafenib will finally solve the clinical dilemma of choosing the proper therapy in this subset of patients.
Supplementary Material
Key Points.
Conventional TACE (cTACE) and drug-eluting-beads TACE (DEB-TACE) demonstrated equal safety profiles.
Survival rates after TACE are similar to patients treated with sorafenib.
Child-Pugh class and tumor burden are reliable predictors of survival.
Acknowledgments
The scientific guarantor of this publication is Jean-Francois Geschwind MD. The authors of this manuscript declare relationships with the following companies. Jean-Francois Geschwind: Philips Medical, Nordion, Biocompatibles/BTG, Bayer HealthCare, DOB, Context Vision, SIR, RSNA; Guerbet
MingDe Lin: Philips Research North America employee.
Boris Gorodetski, Julius Chapiro, Ruediger Schernthaner, Rafael Duran, Howard Lee, David Lenis, Elizabeth A. Stuart, Bareng Aletta Sanny Nonyane, Vasily Pekurovsky, Anobel Tamrazi, and Timothy M. Pawlik: no relevant relationship to a company.
This study was funded by NIH/NCI R01 CA160771, P30 CA006973, NCRR UL1 RR 025005, Philips Research North America, Briarcliff Manor, New York, Ernst Ludwig Ehrlich Foundation, Gerhard C. Starck Foundation and the Rolf W. Gunther Foundation for Radiological Science. There are no financial or other conflicts of interest in relation to this manuscript. David Lenis, Elizabeth A. Stuart PhD and Bareng Aletta Sanny Nonyane PhD kindly provided statistical advice for this manuscript. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Some study subjects or cohorts have been previously presented at the RSNA conference 2014 and at the CIRSE conference in Lisbon 2015.
Abbreviations
- BCLC
Barcelona Clinic Liver Cancer
- CT
Computed tomography
- cTACE
Conventional trans-arterial chemoembolization
- CTCAE
National Cancer Institute Common Terminology Criteria for Adverse Events
- DEB-TACE
Drug-eluting beads TACE
- EASL
European Association for the Study of the Liver
- ECOG
PS Eastern Cooperative Oncology Group performance status
- HCC
Hepatocellular carcinoma
- HKLC
Hong Kong Liver Cancer
- IAT
Intra-arterial therapy
- MOS
Median overall survival
- MRI
Magnet resonance imaging
- PD
Progressive disease
- PES
Post-embolic syndrome
- PVT
Portal-venous thrombosis
- SD
Stable disease
- SHARP
Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol
- TACE
Trans-arterial chemoembolization
- Y90 RE
Yttrium 90 radioembolization
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00330-016-4445-9) contains supplementary material, which is available to authorized users.
Gorodetski, et al. Survival outcomes in patients with advanced-stage HCC and portal vein thrombosis: Comparison between conventional and drug-eluting beads TACE. The abstract was presented at the 100th Scientific Assembly and Annual Meeting of the Radiological Society of North America. Chicago, Illinois, 30 November – 5 December 2014.
Gorodetski, et al. Is trans-arterial chemoembolization safe in patients with advanced to end-stage HCC and portal vein invasion? Comparison between conventional and drug-eluting beads TACE. The abstract was presented at the last CIRSE meeting. Lisbon, Portugal, 26 – 30 September 2015.
Methodology: retrospective, observational, performed at one institution.
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Global battle against cancer won’t be won with treatment alone–effective prevention measures urgently needed to prevent cancer crisis. Cent Eur J Public Health. 2014;22:23, 28. [PubMed] [Google Scholar]
- 4.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz RE, Abou-Alfa GK, Geschwind JF, et al. Nonoperative therapies for combined modality treatment of hepatocellular cancer: expert consensus statement. HPB (Oxford) 2010;12:313–320. doi: 10.1111/j.1477-2574.2010.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M, American Association for the Study of Liver D Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapiro J, Tacher V, Geschwind JF. Intraarterial therapies for primary liver cancer: state of the art. Expert Rev Anticancer Ther. 2013;13:1157–1167. doi: 10.1586/14737140.2013.845528. [DOI] [PubMed] [Google Scholar]
- 8.Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691–1700.e1693. doi: 10.1053/j.gastro.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 10.Villanueva A, Hernandez-Gea V, Llovet JM. Medical therapies for hepatocellular carcinoma: a critical view of the evidence. Nat Rev Gastroenterol Hepatol. 2013;10:34–42. doi: 10.1038/nrgastro.2012.199. [DOI] [PubMed] [Google Scholar]
- 11.Kalva SP, Pectasides M, Liu R, et al. Safety and effectiveness of chemoembolization with drug-eluting beads for advanced-stage hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013 doi: 10.1007/s00270-013-0654-7. [DOI] [PubMed] [Google Scholar]
- 12.Prajapati HJ, Dhanasekaran R, El-Rayes BF, et al. Safety and efficacy of doxorubicin drug-eluting bead transarterial chemoembolization in patients with advanced hepatocellular carcinoma. J Vasc Interv Radiol. 2013;24:307–315. doi: 10.1016/j.jvir.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Georgiades CS, Hong K, D’Angelo M, Geschwind JF. Safety and efficacy of transarterial chemoembolization in patients with unresectable hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2005;16:1653–1659. doi: 10.1097/01.RVI.0000182185.47500.7A. [DOI] [PubMed] [Google Scholar]
- 14.Chung GE, Lee JH, Kim HY, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258:627–634. doi: 10.1148/radiol.10101058. [DOI] [PubMed] [Google Scholar]
- 15.Chapiro J, Geschwind JF. Hepatocellular carcinoma: have we finally found the ultimate staging system for HCC? Nat Rev Gastroenterol Hepatol. 2014;11:334–336. doi: 10.1038/nrgastro.2014.67. [DOI] [PubMed] [Google Scholar]
- 16.Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reyes DK, Vossen JA, Kamel IR, et al. Single-center phase II trial of transarterial chemoembolization with drug-eluting beads for patients with unresectable hepatocellular carcinoma: initial experience in the United States. Cancer J. 2009;15:526–532. doi: 10.1097/PPO.0b013e3181c5214b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yau T, Chan P, Ng KK, et al. Phase 2 open-label study of single-agent sorafenib in treating advanced hepatocellular carcinoma in a hepatitis B-endemic Asian population: presence of lung metastasis predicts poor response. Cancer. 2009;115:428–436. doi: 10.1002/cncr.24029. [DOI] [PubMed] [Google Scholar]
- 19.Cheng AL, Guan Z, Chen Z, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur J Cancer. 2012;48:1452–1465. doi: 10.1016/j.ejca.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821–829. doi: 10.1016/j.jhep.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Edeline J, Crouzet L, Campillo-Gimenez B, et al. Selective internal radiation therapy compared with sorafenib for hepatocellular carcinoma with portal vein thrombosis. Eur J Nucl Med Mol Imaging. 2015 doi: 10.1007/s00259-015-3210-7. [DOI] [PubMed] [Google Scholar]
- 22.de la Torre M, Buades-Mateu J, de la Rosa PA, et al. A comparison of survival in patients with hepatocellular carcinoma and portal vein invasion treated by radioembolization or Sorafenib. Liver Int. 2016 doi: 10.1111/liv.13098. [DOI] [PubMed] [Google Scholar]
- 23.Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 24.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Hilgard P, Hamami M, Fouly AE, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741–1749. doi: 10.1002/hep.23944. [DOI] [PubMed] [Google Scholar]
- 26.Memon K, Kulik L, Lewandowski RJ, et al. Radioembolization for hepatocellular carcinoma with portal vein thrombosis: impact of liver function on systemic treatment options at disease progression. J Hepatol. 2013;58:73–80. doi: 10.1016/j.jhep.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biederman DM, Titano JJ, Tabori NE, et al. Outcomes of radioembolization in the treatment of hepatocellular carcinoma with portal vein invasion: resin versus glass microspheres. J Vasc Interv Radiol. 2016 doi: 10.1016/j.jvir.2016.01.147. [DOI] [PubMed] [Google Scholar]
- 28.Carr BI, Kondragunta V, Buch SC, Branch RA. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer. 2010;116:1305–1314. doi: 10.1002/cncr.24884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79:2087–2094. [PubMed] [Google Scholar]
- 30.Luo J, Guo RP, Lai EC, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413–420. doi: 10.1245/s10434-010-1321-8. [DOI] [PubMed] [Google Scholar]
- 31.Niu ZJ, Ma YL, Kang P, et al. Transarterial chemoembolization compared with conservative treatment for advanced hepatocellular carcinoma with portal vein tumor thrombus: using a new classification. Med Oncol. 2012;29:2992–2997. doi: 10.1007/s12032-011-0145-0. [DOI] [PubMed] [Google Scholar]
- 32.Chung JW, Park JH, Han JK, Choi BI, Han MC. Hepatocellular carcinoma and portal vein invasion: results of treatment with transcatheter oily chemoembolization. AJR Am J Roentgenol. 1995;165:315–321. doi: 10.2214/ajr.165.2.7618547. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Zhang C, Zhao Y, et al. Transarterial chemoembolization for the treatment of advanced hepatocellular carcinoma with portal vein tumorthrombosis: prognostic factors in a single-center study of 188 patients. Biomed Res Int. 2014;2014:194278. doi: 10.1155/2014/194278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu K, Chen J, Lai L, et al. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib-A retrospective controlled study. Radiology. 2014 doi: 10.1148/radiol.14131946:131946. [DOI] [PubMed] [Google Scholar]
- 35.Huang M, Lin Q, Wang H, et al. Survival benefit of chemoembolization plus Iodine125 seed implantation in unresectable hepatitis B-related hepatocellular carcinoma with PVTT: a retrospective matched cohort study. Eur Radiol. 2016 doi: 10.1007/s00330-015-4198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li XL, Guo WX, Hong XD, et al. Efficacy of the treatment of transarterial chemoembolization combined with radiotherapy for hepatocellular carcinoma with portal vein tumor thrombus: a propensity score analysis. Hepatol Res. 2016 doi: 10.1111/hepr.12657. [DOI] [PubMed] [Google Scholar]
- 37.McEvoy SH, McCarthy CJ, Lavelle LP, et al. Hepatocellular carcinoma: illustrated guide to systematic radiologic diagnosis and staging according to guidelines of the American Association for the Study of Liver Diseases. Radiographics. 2013;33:1653–1668. doi: 10.1148/rg.336125104. [DOI] [PubMed] [Google Scholar]
- 38.Therneau T. A package for survival analysis in S, pp R package version 2. 2014:37–37. [Google Scholar]
- 39.Uraki J, Yamakado K, Nakatsuka A, Takeda K. Transcatheter hepatic arterial chemoembolization for hepatocellular carcinoma invading the portal veins: therapeutic effects and prognostic factors. Eur J Radiol. 2004;51:12–18. doi: 10.1016/S0720-048X(03)00219-5. [DOI] [PubMed] [Google Scholar]
- 40.Sato K, Lewandowski RJ, Bui JT, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol. 2006;29:522–529. doi: 10.1007/s00270-005-0171-4. [DOI] [PubMed] [Google Scholar]
- 41.Pellerin O, Lin M, Bhagat N, Shao W, Geschwind JF. Can C-arm cone-beam CT detect a micro-embolic effect after TheraSphere radioembolization of neuroendocrine and carcinoid liver metastasis? Cancer Biother Radiopharm. 2013;28:459–465. doi: 10.1089/cbr.2012.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Malenstein H, Maleux G, Vandecaveye V, et al. A randomized phase II study of drug-eluting beads versus transarterial chemoembolization for unresectable hepatocellular carcinoma. Onkologie. 2011;34:368–376. doi: 10.1159/000329602. [DOI] [PubMed] [Google Scholar]
- 43.Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug-eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 44.Wang JH, Changchien CS, Hu TH, et al. The efficacy of treatment schedules according to Barcelona Clinic Liver Cancer staging for hepatocellular carcinoma - survival analysis of 3892 patients. Eur J Cancer. 2008;44:1000–1006. doi: 10.1016/j.ejca.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 45.de Baere T, Denys A, Briquet R, Chevallier P, Dufaux J, Roche A. Modification of arterial and portal hemodynamics after injection of iodized oils and different emulsions of iodized oils in the hepatic artery: an experimental study. J Vasc Interv Radiol. 1998;9:305–310. doi: 10.1016/s1051-0443(98)70273-8. [DOI] [PubMed] [Google Scholar]
- 46.Kan Z, Ivancev K, Lunderquist A. Peribiliary plexa-important pathways for shunting of iodized oil and silicon rubber solution from the hepatic artery to the portal vein. An experimental study in rats. Investig Radiol. 1994;29:671–676. doi: 10.1097/00004424-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Kan Z. Dynamic study of iodized oil in the liver and blood supply to hepatic tumors. An experimental investigation in several animal species. Acta Radiol Suppl. 1996;408:1–25. [PubMed] [Google Scholar]
- 48.Shin SW. The current practice of transarterial chemoembolization for the treatment of hepatocellular carcinoma. Korean J Radiol. 2009;10:425–434. doi: 10.3348/kjr.2009.10.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590–599. doi: 10.1148/radiol.12111550. [DOI] [PubMed] [Google Scholar]
- 50.Ozenne V, Paradis V, Pernot S, et al. Tolerance and outcome of patients with unresectable hepatocellular carcinoma treated with sorafenib. Eur J Gastroenterol Hepatol. 2010;22:1106–1110. doi: 10.1097/MEG.0b013e3283386053. [DOI] [PubMed] [Google Scholar]
- 51.McDonald RJ, McDonald JS, Kallmes DF, Carter RE. Behind the numbers: propensity score analysis-a primer for the diagnostic radiologist. Radiology. 2013;269:640–645. doi: 10.1148/radiol.13131465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


