Abstract
miR-20b is a member of the miR-106a-363 gene cluster, which has been shown to play an important role in a variety of diseases, including cancer, inflammation, and autoimmune diseases. Our previous study indicated that miR-20b has an inhibitory effect on airway inflammation in asthmatic mice, but the exact mechanism is unclear. In this study, we report that the ratio of CD11b+Ly6G+Ly6Clow cells, but not the amount of CD11b+Ly6C+Ly6G– cells, was increased in the lung tissue of asthmatic mice after intranasal instillation with miR-20b mimics, while Th2-type cytokines (interleukin (IL)-4 and IL-13) were significantly decreased in the bronchoalveolar lavage fluid. In addition, the transcription factor CREB regulated the expression of miR-20b. Our findings suggest that miR-20b can induce the accumulation of myeloid-derived suppressor cells in the lungs of asthmatic mice, which may be a mechanism by which miR-20b inhibits airway inflammation in asthmatic mice. Thus, miR-20b may be used as a target for the effective treatment of asthma in the future.
Keywords: asthma, MiR-20b, myeloid-derived suppressor cells (MDSCs), allergic airway inflammation
Introduction
Bronchial asthma (here referred to as asthma) is chronic airway inflammation caused by many kinds of cells and inflammatory factors. Airway hyper-responsiveness, chronic inflammation, and airway remodeling are characteristic features of asthma [1, 2]. Epidemiological studies have found that the incidence and mortality of asthma are increasing year by year, but its pathogenesis is still unclear at present [3]. MicroRNAs (miRNAs) are non-encoding RNAs which are about 22 nucleotides in length. They regulate the expression of target genes by inhibiting their translation or causing mRNA degradation [4]. An increasing number of studies has found that many miRNAs may participate in asthma pathogenesis [5]. In an asthmatic mouse model, the upregulation of miR-221 and miR-485-3p may play an important role in the pathogenesis of asthma by downregulating the expression of Spre-2 [6]. Collison et al. demonstrated that the expression of miR-126 was upregulated in the airway wall tissues of asthmatic mice, and blocking miR-126 significantly reduced airway eosinophil cell infiltration [7]. Panganiban et al. showed that the expression of miR-1248 was upregulated in the serum of asthmatic patients, and it positively regulates the expression of interleukin (IL)-5, which is a key factor for chemotaxis, survival, and maturation of eosinophils [8]. In severe asthma, blood CD8+ T cells are activated at the same time, accompanied by reduced miR-146a/b and miR-28-5p expression [9]. In our previous study, we found that the expression of miR-20b in the lung tissues of asthmatic mice was significantly lower than that of normal mice [10]. In addition, miR-20b inhibited airway inflammation in asthmatic mice, but the mechanism remains unclear [11]. Since myeloid-derived suppressor cells (MDSCs) effectively inhibit airway inflammation in asthmatic mice [12], in this study we analyzed the effect of miR-20b on MDSCs content in the lung tissue of asthmatic mice.
Material and methods
Mice
Female BALB/c mice (2- to 3-week-old) were purchased from the Center of Experimental Animals of Bengbu Medical College. This study was approved by the Animal Care and Ethics Committee of Bengbu Medical College.
Establishment of experimental asthma model and treatment
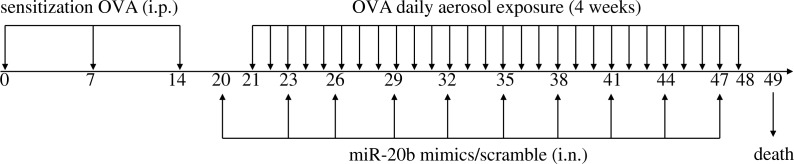
Female BALB/c mice were randomly divided into four groups, a normal control group (Control), an asthmatic group (Asthma), a miR-20b-mimic treatment group (MiR-20b mimic) and a scrambled miR-20b treatment group (MiR-20b scrambled), with seven mice in each group. For all groups except the Control group, mice were sensitized by intraperitoneal injection with 200 μl liquid (including 50 μg of OVA and 2 mg of aluminum hydroxide) on days 0, 7, and 14. On day 21 from the start of the experiment, the mice were placed in an atomization inhalation box and were challenged with 5% OVA solution inhalation with nebulization for 30 min, continuously for 4 weeks. In addition, the MiR-20b mimic group was treated with 20 μg miR-20b mimics in 40 μl doses by nasal drip starting from the 20th day, once every three days for a total of 10 doses. The MiR-20b scrambled group received scrambled miR-20b instillation to replace the treatment with miR-20b mimics (Fig.1).
Fig. 1.
Experimental procedure in this study. BALB/c mice were sensitized by intraperitoneal injection of OVA bound to Imject® Alum and were challenged with 5% OVA solution inhalation. miR-20b mimics or scramble were administered via intranasal instillation (see Materials and methods for detailed explanations)
Preparation of bronchoalveolar lavage fluid (BALF)
On day 49, the mice in each group were sacrificed and tracheal intubations were performed. Mice were lavaged 6 times with 0.8 ml PBS. The BALF obtained (in total about 4.8 ml) was centrifuged at 500 × g for 5 min and the supernatant was stored at –20°C for later use in an enzyme-linked immunosorbent assay (ELISA).
Oligonucleotides, siRNAs, and primers
MiR-20b mimics, scrambled miR-20b, CREB siRNA, and CREB control siRNA were obtained from Genepharma (Shanghai, China). The miRNA sequences used were as follows: miRNA-20b mimics, sense: 5’-CAA AGU GCU CAU AGU GCA GGU AG-3’, antisense: 5’-ACC UGC ACU AUG AGC ACU UUG UU-3’; scrambled miR-20b, sense: 5’-UUC UCC GAA CGU GUC ACG UTT-3’, antisense: 5’-ACG UGA CAC GUU CGG AGA ATT-3’; CREB siRNA, sense: 5’-GUC UCC ACA AGU CCA AAC ATT-3’, antisense: 5’-UGU UUG GAC UUG UGG AGA CTT-3’; and CREB control siRNA, sense: 5’-UUC UCC GAA CGU GUC ACG UTT-3’, antisense: 5’-ACG UGA CAC GUU CGG AGA ATT-3’. PCR forward and reverse primers were synthesized by Sangon Biotech (Shanghai, China). The primer sequences used were: GAPDH primer, sense: 5’-GGC AAA TTC AAC GGC ACA-3’, antisense: 5’-TCC ACG ACA TAC TCA GCA CC-3’; CREB primer, sense: 5’-TGC CAC ATT AGC CCA GGT A-3’, antisense: 5’-GGG AGG ACG CCA TAA CAA CT-3’; miR-20b primer, sense: 5’-ATG CCA AAG TGC TCA TAG TG-3’, antisense: 5’-GTG CAG GGT CCG AGG T-3’; miR-20b RT primer: 5’-GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACC TAC CT-3’; U6 primer, sense: 5’-CTCGCT TCG GCA GCA CA-3’, antisense: 5’-AAC GCT TCA CGA ATT TGC GT-3’; and U6 RT primer: 5’-AAC GCT TCA CGA ATT TGC GT-3’.
Transient transfection
CREB siRNA and control siRNA (160 nM) were transfected using Lipofectamine 2000 (Invitrogen) according to the instructions of the manufacturer. A murine blood macrophage cell line (RAW 264.7 cells; 7.2 × 105 cells/well) was seeded into a six-well plate, and the following day, the transfected cells reached 70-90% confluence. The siRNAs and Lipofectamine 2000 were diluted with Opti-MEM with thorough mixing at room temperature for 20 min. The RAW 264.7 cells were incubated with this mixture for 48 h in standard medium. Finally, the cells were harvested for specific experiments.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using TRIZOL (Invitrogen). cDNA was synthesized with a TransScript First-strand cDNA Synthesis SuperMix kit from TransGen. The reaction mix included total RNA (500 ng), 1 μl anchored oligo (dT), 10 μl 2 × TS Reaction Mix, and 1 μl TransScript RT/RI Enzyme Mix (the anchored oligo (dT) was replaced with the RT primer corresponding to the target gene when the miRNA cDNA first-strand was synthesized). The reaction conditions were 30 min at 42°C then 85°C for 5 min. Quantitative PCR (qPCR) was performed with the StepOnePlus System (ABI) using the TransStartTM EcoGreen qPCR SuperMix (TransGen). The amplification conditions were 94°C for 30 s, followed by 40 cycles of 94°C for 5 s, and 60°C for 30 s. U6 RNA or GAPDH were used as endogenous controls for miRNA or other mRNA levels. The relative gene expression was analyzed using the 2ŻΔΔCT method.
FACS analysis
On day 49, the lung tissues from each group were obtained. Single-cell suspensions were prepared from these lung tissues and incubated with fluorescein-conjugated antibodies for 30 min at 4°C in the dark. The stained cells were analyzed using a flow cytometer (BD FACSCaliber).
ELISA
The amount of cytokines in the serum or the BALF was determined with commercial sandwich ELISA kits (CUSABIO) according to the manufacturer’s instructions.
Western blot analysis
Briefly, RAW 264.7 cells were lysed with NP-40 lysis buffer with 1 mM PMSF. Cell lysates were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were blocked with 5% BSA in TBST and probed with the respective antibodies. The blots were detected using enhanced chemiluminescence according to the manufacturer’s protocol (Beyotime biotechnology).
Statistical analysis
Data are expressed as mean ± standard deviation. SPSS16.0 was used to perform single-factor analysis of variance for multiple groups. A P-value < 0.05 was considered statistically significant.
Results
MiR-20b suppresses Th2 cytokine production in the BALF of asthmatic mice
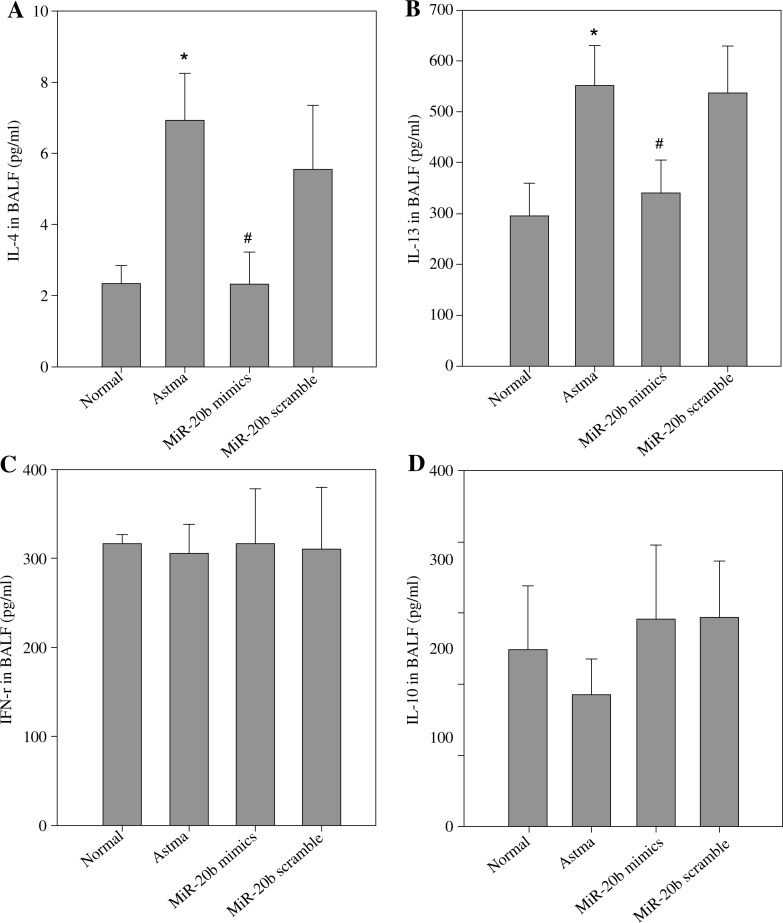
To understand the mechanism by which miR-20b inhibits allergic airway inflammation, we studied the effect of miR-20b on the production of Th1/Th2-type cytokines in mouse BALF. The results are shown in Fig. 2. Compared with the normal control group, the levels of the Th2-type cytokines IL-4 and IL-13 were significantly increased in the BALF of the asthmatic mice. However, the Th1-type cytokine IFN-γ was not significantly decreased in the asthmatic mice. The secretion of IL-13 and IL-4 was significantly decreased after treatment with miR-20b mimics in asthmatic mice (p < 0.01). In contrast, the production of IL-10 in each group was not significantly different in this experimental system.
Fig. 2.
miR-20b suppresses Th2 cytokine production in the BALF of asthmatic mice. BALF was obtained on day 49 after baseline. The concentration of Th1/Th2 cytokines IL-4 (A), IL-13 (B), IFN-γ (C), and IL-10 (D) in the supernatant of the BALF (pg/ml) was assayed by ELISA. “Normal” refers to normal control mice. “Asthma” means asthma model mice. “miR-20b mimics” represents asthmatic mice treated with miR-20b mimics. “miR-20b scramble” refers to asthmatic mice treated with miR-20b scramble. Data are expressed as mean ± standard deviation;* p < 0.01 compared with the Normal group; #p < 0.01 compared with the Asthma group
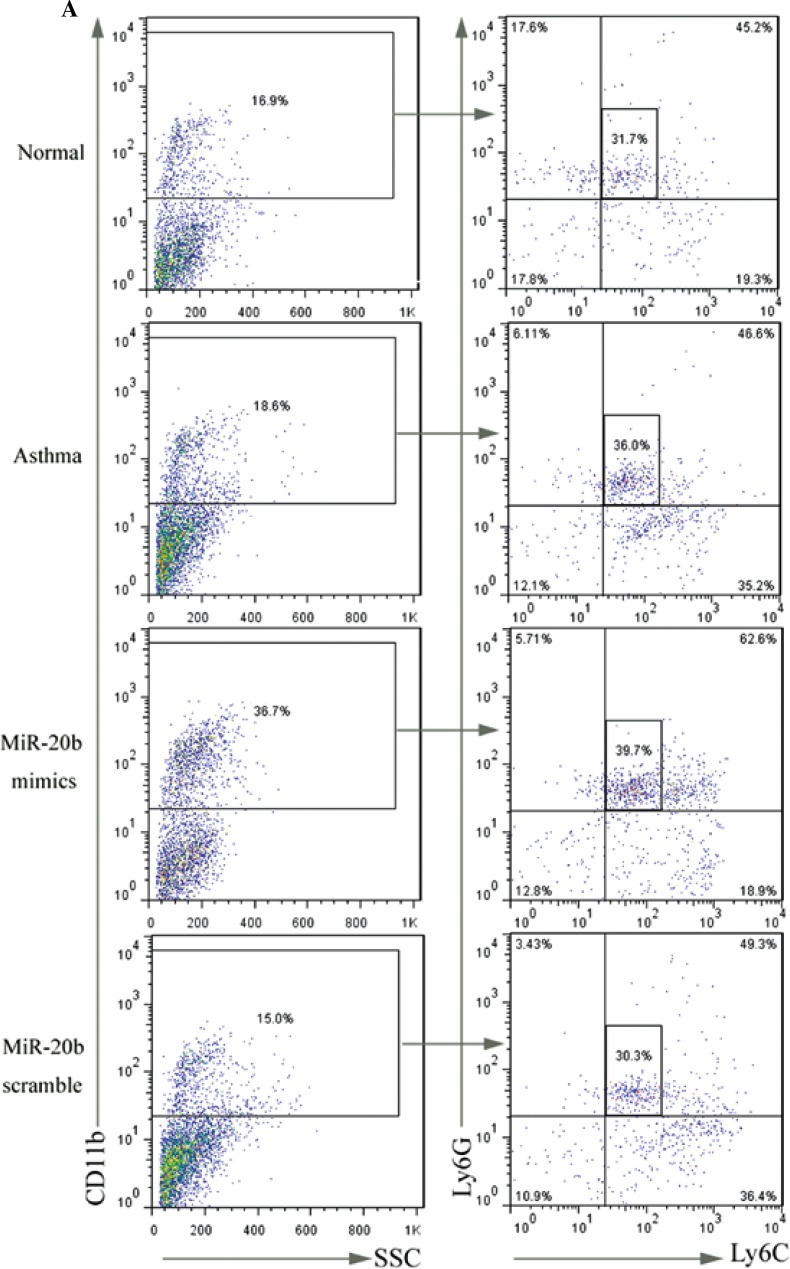
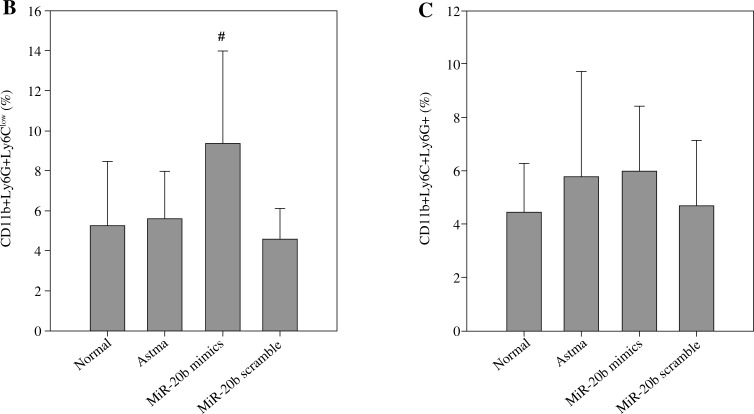
MiR-20b promotes the accumulation of CD11b+Ly6G+Ly6Clow MDSCs in asthmatic mice
Since MDSCs have significant immunosuppressive activity, their relationship with asthma has recently attracted attention. To discern whether the inhibitory effect of miR-20b on airway inflammation involves MDSCs, the MDSC profiles in the lung tissues of mice in each group were assayed (Fig. 3). The results showed that there was not a significant increase of MDSCs in the asthma group compared with the normal group. After treatment with miR-20b mimics, the CD11b+Ly6G+Ly6Clow MDSC ratio was significantly elevated in asthmatic mice (p < 0.05), while the increase in CD11b+Ly6C+Ly6G- MDSCs was not statistically significant. Treatment with miR-20b-scramble did not have a significant effect on the content of MDSCs in the lung tissues of asthmatic mice.
Fig. 3.
miR-20b promotes the accumulation of CD11b+Ly6G+Ly6Clow MDSCs in asthmatic mice. Lung tissues of mice were surgically removed on day 49 after baseline. Single-cell suspensions were prepared and stained with FITC-Ly6G, PE-Ly6C, and PE-cy5-CD11b. Stained cells were analyzed using flow cytometry (A). The proportion of CD-11b+Ly6G+Ly6Clow cells (B) and CD11b+Ly6C+Ly6G- cells (C) in lung tissues were calculated. Data are expressed as mean ± standard deviation; #p < 0.05 compared with the Asthma group
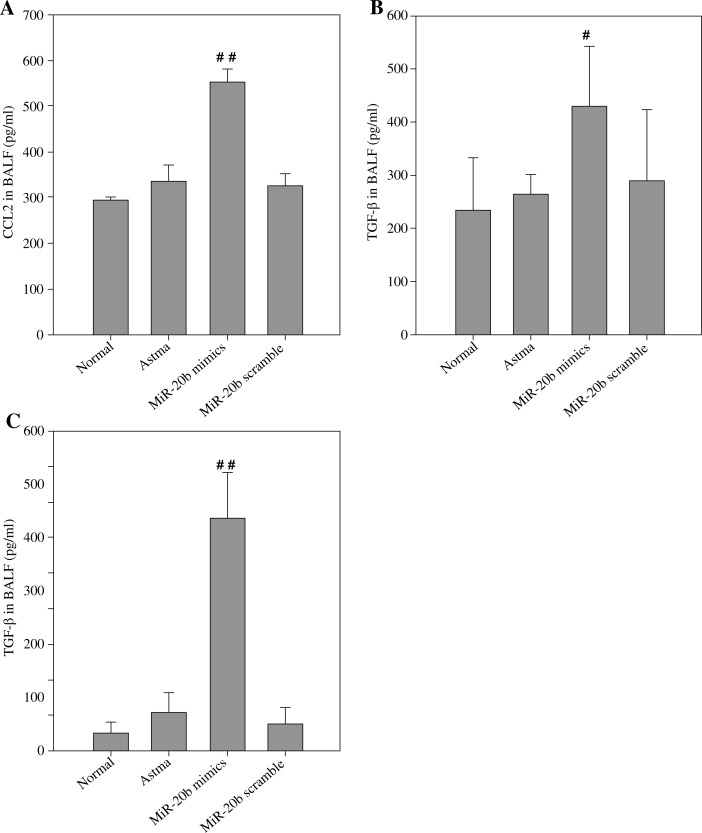
MiR-20b increases CCL2 and TGF-β in asthmatic mice
In this study, the CCL2 content in each group was detected, and the results are shown in Fig. 4A. There was not a significant difference in CCL2 concentration between the asthmatic group and the normal group, while CCL2 levels in BALF were significantly higher after asthmatic mice treatment with miR-20b mimics. Therefore, miR-20b induced the accumulation of MDSCs accompanied by an increase in CCL2 concentration in the lungs of asthmatic mice. Because TGF-β is an important effector molecule of MDSCs, the concentration of TGF-β in the BALF and serum of each group of mice was assayed (Fig. 4B, C). As expected, relative to the other three groups, the concentration of TGF-β, whether in the serum or in the BALF, was increased in the miR-20b-mimic treatment group.
Fig. 4.
miR-20b increases CCL2 and TGF-β in asthmatic mice. On day 49, CCL2 (A) and TGF-β (B) in the BALF and TGF-β in the serum (C) of each group of mice were measured using ELISA. Data are expressed as mean ± standard deviation; #p < 0.05, # #p < 0.01 compared with the Asthma group
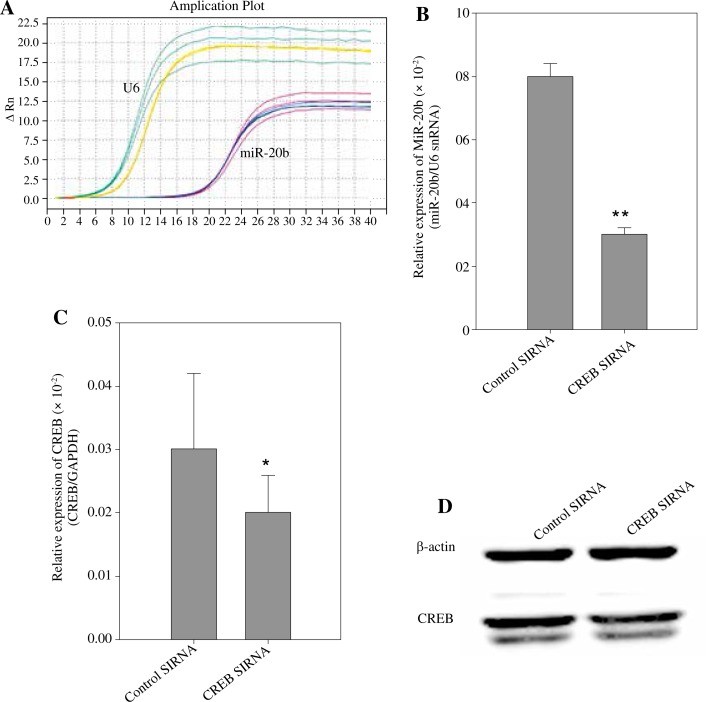
CREB regulates miR-20b expression
At present, there have been few studies on the regulation of miR-20b expression. We used two bioinformatics software packages, P-MATCH 1.0 (http://www.gene-regulation.com/cgi-bin/pub/programs/pmatch/bin/p-match.cgi) and AliBaba2 (http://www.gene-regulation.com/pub/programs/alibaba2/index.html), to predict the transcription factors that might be involved in the regulation of miR-20b expression, in which the CREB score was higher. Thus, we attempted to verify whether CREB regulates the expression of miR-20b. The expression of miR-20b in RAW 264.7 cells transfected with CREB siRNA was analyzed by qRT-PCR analysis (Fig. 5A, B). CREB siRNA decreased the miR-20b level to 0.375-fold of the control (p < 0.01). To confirm the activity of CREB siRNA, we examined the mRNA and protein level of CREB in RAW 264.7 cells after transfection. The level of CREB mRNA decreased by 66% after CREB siRNA transfection for 48 h (Fig. 5C). The expression of the CREB protein was also decreased by 75% (Fig. 5D). These results indicate that the expression of miR-20b is regulated by CREB upstream in RAW 264.7 cells.
Fig. 5.
CREB regulates miR-20b expression. RAW 264.7 cells were transfected with either siRNA control or CREB siRNA for 48 h. To detect miR-20b levels, qRT-PCR was used (A) and the relative expression of miR-20b to U6 snRNA was calculated (B). The expression of CREB mRNA and protein were detected by qPCR (C) and Western blot (D) analyses. Data are expressed as the mean ± standard deviation of four independent experiments; *p < 0.05, **p < 0.01 compared with the Control group
Discussion
Myeloid-derived suppressor cells are a group of myeloid precursor cells which fail to differentiate into mature immune cells. Having prominent immunosuppressive activity, MDSCs can significantly inhibit the function of a variety of T cells [13, 14]. Myeloid-derived suppressor cells can be divided into different subsets based on their surface molecules. In humans, MDSCs can be subdivided into the CD11b+CD33+HLA-DR−CD14+ and CD11b+CD15+HLA-DR−CD14− subgroups. In mice, MDSCs can be further subdivided into CD11b+Ly6G+Ly6Clow and CD11b+Ly6C+Ly6G− subsets according to Ly-6C/G expression. The former group exhibits a granulocytic phenotype (G-MDSC), while the latter displays a monocytic phenotype (M-MDSC) [15, 16].
In this study, the proportion of CD11b+Ly6G+Ly6Clow MDSCs increased in lung tissue from asthmatic mice after administration of nasal drops containing miR-20b mimics, but the ratio of CD11b+Ly6C+Ly6G− MDSCs was not changed. The latest research shows that many miRNAs are related to the biological functions of MDSCs, such as chemotaxis, aggregation, and functional activities [17]. Liu et al. demonstrated that miR-494 may activate the Akt pathway by targeting PTEN to regulate the accumulation and functional activity of MDSCs. In addition, miR-494 mediated the chemotaxis of MDSCs by CXCR4 chemokines in tumor tissues [18]. Li et al. showed that miR-155 and miR-21 increased GM-CSF and IL-6-induced bone marrow cell-derived MDSCs, which includes both G-MDSC and M-MDSC types. Furthermore, miR-155 and miR-21 activated STAT3 by targeting SHTP-1 and PTEN to synergistically induce the generation of MDSCs [19]. Mei et al. demonstrated that miR-200c contributed to the expansion and immunosuppressive activity of MDSCs in a mouse lung cancer and melanoma model [20]. Our previous study indicated that CCL2 signaling mediates the migration of MDSCs into the lung. In this study, we found that miR-20b can induce an increase in CCL2 concentration in the lungs of asthmatic mice [12]. Therefore, miR-20b may induce the accumulation of MDSCs by upregulating CCL2 chemokines in the lungs of asthmatic mice.
Once activated by inflammation- or tumor-driven factors, MDSCs can release a variety of biologically active mediators, including inducible nitric oxide synthase (iNOS), arginase 1, reactive oxygen species (ROS), IL-10, and transforming growth factor β (TGF-β) to inhibit the function of immune cells [21, 22]. Our preliminary results indicate that tumor-derived MDSCs inhibit the allergic Th2 response in a TGF-β-dependent manner. In this study, we also found that the elevation of MDSCs induced by miR-20b was accompanied by an increase in TGF-β in BALF and blood. Thus, miR-20b may inhibit airway inflammation in a TGF-β-dependent manner by inducing MDSCs in asthmatic mice.
Th1/Th2 cytokines play a key role in regulating airway inflammation in asthmatic mice [23]. It is noteworthy that the administration of miR-20b suppressed Th2 cytokines in the BALF of asthmatic mice in the current study. Recent studies have shown that miRNAs play an important role in regulating the balance of the Th1/Th2 immune response [24]. Lu et al. showed that lung eosinophil infiltration was decreased and the Th1-type cytokine IFN-γ was significantly increased when miR-21 was blocked in a murine asthma model. Meanwhile, DC with miR-21 deficiency increased the production of IL-12 after stimulation with LPS. miR-21-deficient mice had significantly increased Th1 delayed-type hypersensitivity [25]. These studies emphasize that miR-21 mainly regulates Th1 response rather than the Th2 reaction. Meanwhile, Mohnle et al. showed that miR-146 phosphorylated STAT4 by targeting PKCε, thus resulting in Th1-cell differentiation [26]. However, our study showed that miR-20b inhibited the Th2-type response in asthmatic mice, but had no effect on Th1-type response. A study by Mattes et al. also showed that the function of Th2 cells was inhibited, and the symptoms of asthma were reduced, in mice with miR-126 ablation, and blocking miR-126 may lead to the increased expression of POU domain class 2 associating factor 1, which can negatively regulate the expression of the Th2-specific regulatory factor GATA3 [27].
This study also demonstrated that the transcription factor CREB can regulate the expression of miR-20b at the upstream level. Since the pathogenesis of asthma is closely related to miRNAs, research on the regulation of miRNAs may shed light on new asthma treatments [28]. At present, research on the regulation of miRNA expression is relatively rare. Recent studies show that the CCCTC-binding factor (CTCF) can not only control the expression of miR-125b1 and miR-375, but can also adjust the level of the miR-290 cluster in tumor cells [29]. Chavali et al. demonstrated that binding of the HMGI/Y protein to matrix attachment regions (MARs) induced histone acetylation, resulting in the expression of the miR-17-92 cluster and individual miRNAs (miR-221, 93, 17, and let-7b) in neuroblastoma cells but not in fibroblasts [30]. Zhang et al. showed that two members of the E-2b family (PEA3 and ELK-1) regulated the expression of miR-200b. Furthermore, ELK-1 inhibited the expression of miR-200b, and PEA3 promoted the expression of miR-200b [31]. Since CREB can regulate the expression of miR-20b, regulating CREB upstream of miR-20b may provide a therapeutic target for asthma treatment.
In summary, we show an important role for miR-20b in the pathogenesis of asthma, inducing the accumulation of CD11b+Ly6G+Ly6Clow cells in lung tissues. This may be a mechanism by which miR-20b suppresses airway inflammation in asthmatic mice. This study provides an experimental basis for the future clinical therapy of asthma using miR-20b as a target point of treatment.
Footnotes
This work was supported by National Science Foundation of China (No. 81273273) and Anhui Provincial Natural Science Foundation (1308085MH114).
The authors declare no conflict of interest.
References
- 1.Pelaia G, Vatrella A, Busceti MT, et al. Cellular mechanisms underlying eosinophilic and neutrophilic airway inflammation in asthma. Mediators Inflamm. 2015;2015:879783. doi: 10.1155/2015/879783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fahy JV. Type 2 inflammation in asthma – present in most, absent in many. Nat Rev Immunol. 2015;15:57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olin JT, Wechsler ME. Asthma: pathogenesis and novel drugs for treatment. BMJ. 2014;349:g5517. doi: 10.1136/bmj.g5517. [DOI] [PubMed] [Google Scholar]
- 4.Su Z, Yang Z, Xu Y, et al. MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget. 2015;11:8474–8490. doi: 10.18632/oncotarget.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perry MM, Adcock IM, Chung KF. Role of microRNAs in allergic asthma: present and future. Curr Opin Allergy Clin Immunol. 2015;2:156–162. doi: 10.1097/ACI.0000000000000147. [DOI] [PubMed] [Google Scholar]
- 6.Liu F, Qin HB, Xu B, et al. Profiling of miRNAs in pediatric asthma: upregulation of miRNA-221 and miRNA-485-3p. Mol Med Rep. 2015;5:1178–1182. doi: 10.3892/mmr.2012.1030. [DOI] [PubMed] [Google Scholar]
- 7.Collison A, Herbert C, Siegle JS, et al. Altered expression of microRNA in the airway wall in chronic asthma: miR-126 as a potential therapeutic target. BMC Pulm Med. 2011;11:29. doi: 10.1186/1471-2466-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panganiban RP, Pinkerton MH, Maru SY, et al. Differential microRNA epression in asthma and the role of miR-1248 in regulation of IL-5. Am J Clin Exp Immunol. 2012;2:154–165. [PMC free article] [PubMed] [Google Scholar]
- 9.Tsitsiou E, Williams AE, Moschos SA, et al. Transcriptome analysis shows activation of circulating CD8+ T cells in patients with severe asthma. J Allergy Clin Immunol. 2012;129:95–103. doi: 10.1016/j.jaci.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Song C, Ma H, Yao C, et al. Alveolar macrophage-derived vascular endothelial growth factor contributes to allergic airway inflammation in a mouse asthma model. Scand J Immunol. 2012;75:599–605. doi: 10.1111/j.1365-3083.2012.02693.x. [DOI] [PubMed] [Google Scholar]
- 11.Ma H, Luo YL, Guo SJ, et al. Inhibitory effect of miR-20b on airway inflammation in asthmatic mice. Nan Fang Yi Ke Da Xue Xue Bao. 2015;35:1463–1466. [PubMed] [Google Scholar]
- 12.Song C, Yuan Y, Wang XM, et al. Passive transfer of tumour-derived MDSCs inhibits asthma-related airway inflammation. Scand J Immunol. 2014;79:98–104. doi: 10.1111/sji.12140. [DOI] [PubMed] [Google Scholar]
- 13.Nagaraj S, Youn JI, Gabrilovich DI. Reciprocal relationship between myeloid-derived suppressor cells and T cells. J Immunol. 2013;191:17–23. doi: 10.4049/jimmunol.1300654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindau D, Gielen P, Kroesen M, et al. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105–115. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang J, Guo W, Liang X. Phenotypes, accumulation, and functions of myeloid-derived suppressor cells and associated treatment strategies in cancer patients. Hum Immunol. 2014;75:1128–1137. doi: 10.1016/j.humimm.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Zhang Y, Kuzel TM, et al. Regulating Tumor Myeloid-Derived Suppressor Cells by MicroRNAs. Cancer Cell Microenviron. 2015;2:e637. doi: 10.14800/ccm.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Lai L, Chen Q, et al. MicroRNA-494 is required for the accumulation and functions of tumor-expanded myeloid-derived suppressorcells via targeting of PTEN. J Immunol. 2012;188:5500–5510. doi: 10.4049/jimmunol.1103505. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Zhang J, Diao W, et al. MicroRNA-155 and MicroRNA-21 promote the expansion of functional myeloid-derived suppressor cells. J Immunol. 2014;192:1034–1043. doi: 10.4049/jimmunol.1301309. [DOI] [PubMed] [Google Scholar]
- 20.Mei S, Xin J, Liu Y, et al. MicroRNA-200c Promotes Suppressive Potential of Myeloid-Derived Suppressor Cells by Modulating PTEN andFOG2 Expression. PLoS One. 2015;10:e0135867. doi: 10.1371/journal.pone.0135867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markowitz J, Wesolowski R, Papenfuss T, et al. Myeloid-derived suppressor cells in breast cancer. Breast Cancer Res Treat. 2013;140:13–21. doi: 10.1007/s10549-013-2618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostanin DV, Bhattacharya D. Myeloid-derived suppressor cells in the inflammatory bowel diseases. Inflamm Bowel Dis. 2013;19:2468–2477. doi: 10.1097/MIB.0b013e3182902b11. [DOI] [PubMed] [Google Scholar]
- 23.Tang F, Wang F, An L, et al. Upregulation of Tim-3 on CD4(+) T cells is associated with Th1/Th2 imbalance in patients with allergic asthma. Int J Clin Exp Med. 2015;8:3809–3816. [PMC free article] [PubMed] [Google Scholar]
- 24.Luo Y, Deng Y, Tao Z, et al. Regulatory effect of microRNA-135a on the Th1/Th2 imbalance in a murine model of allergic rhinitis. Exp Ther Med. 2014;8:1105–1110. doi: 10.3892/etm.2014.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu TX, Hartner J, Lim EJ, et al. MicroRNA-21 limits in vivo immune response-mediated activation of the IL-12/IFN-gamma pathway, Th1polarization, and the severity of delayed-type hypersensitivity. J Immunol. 2011;187:3362–3373. doi: 10.4049/jimmunol.1101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Möhnle P, Schütz SV, van der Heide V, et al. MicroRNA-146a controls Th1-cell differentiation of human CD4+ T lymphocytes by targeting PRKCε. Eur J Immunol. 2015;45:260–272. doi: 10.1002/eji.201444667. [DOI] [PubMed] [Google Scholar]
- 27.Mattes J, Collison A, Plank M, et al. Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proc Natl Acad Sci U S A. 2009;106:18704–18709. doi: 10.1073/pnas.0905063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kai W, Qian XU, Qun WU. MicroRNAs and Asthma Regulation. Iran J Allergy Asthma Immunol. 2015;14:120–125. [PubMed] [Google Scholar]
- 29.Saito Y, Saito H. Role of CTCF in the regulation of microRNA expression. Front Genet. 2012;3:186. doi: 10.3389/fgene.2012.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chavali PL, Funa K, Chavali S, et al. Cis-regulation of microRNA expression by scaffold/matrix-attachment regions. Nucleic Acids Res. 2011;39:6908–6918. doi: 10.1093/nar/gkr303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Zhang B, Gao J, et al. Regulation of the microRNA 200b (miRNA-200b) by transcriptional regulators PEA3 and ELK-1 protein affectsexpression of Pin1 protein to control anoikis. J Biol Chem. 2013;288:32742–32752. doi: 10.1074/jbc.M113.478016. [DOI] [PMC free article] [PubMed] [Google Scholar]