Figure 4.
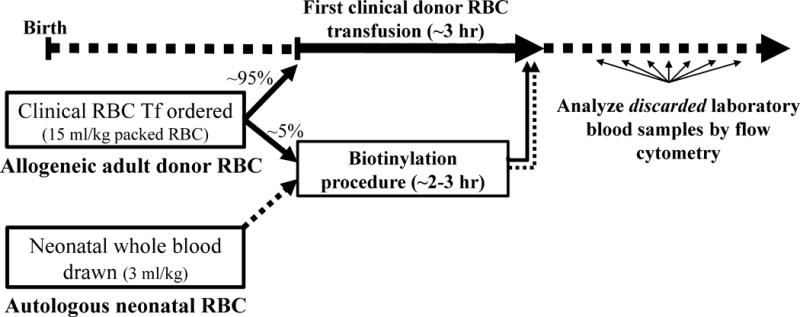
Study protocol diagram of RBC biotinylation and population enumeration by flow cytometry. Allogeneic adult donor RBCs and autologous neonatal RBCs were labeled at discreet biotin density levels and and transfused to VLBW anemic study subjects at the end of the first (unlabeled) RBC transfusion given to treat anemia. Post-transfusion blood samples leftover from laboratory testing were analyzed by flow cytometry to determine the fraction of biotin-labeled adult and neonatal RBCs that remained in circulation.
