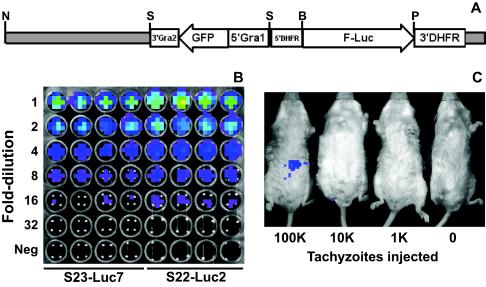
FIG. 1.
(A) Construct used to generate luciferase-expressing T. gondii clones S23-luc7 and S22-luc2. pDHFR-Luc (obtained from David Roos, University of Pennsylvania), a plasmid with a pBluescript KS(−) backbone and the firefly luciferase gene driven by the T. gondii DHFR promoter, was modified by addition of a GFP expression cassette driven by the GRA1 promoter. The plasmid was linearized with NotI prior to transfection. Enzyme abbreviations: N, NotI; S, SalI; B, BglII; P, PstI. (B) Dilution of S23-luc7 and S22-luc2 tachyzoites, demonstrating the similar levels of light production in the presence of d-luciferin. Freshly harvested tachyzoites were quantified and serially diluted in twofold increments in 96-well plates with black walls in quadruplicate. d-Luciferin was added, and each plate was imaged 10 min later. The topmost wells contained 5 × 105 tachyzoites. In the pseudocolor images the luciferase photon intensity ranges from the lowest intensity (blue) to the highest intensity (red). (C) S22-luc2 tachyzoites were serially diluted in 10-fold increments, and different amounts were injected intraperitoneally into BALB/c mice. Luciferin was injected immediately after this, and 10 min later mice were imaged as described in Materials and Methods. The minimum number of tachyzoites necessary to detect a bioluminescent signal greater than the background signal in these experiments was between 10,000 and 100,000.