Abstract
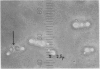
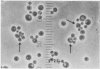
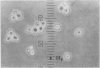
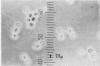
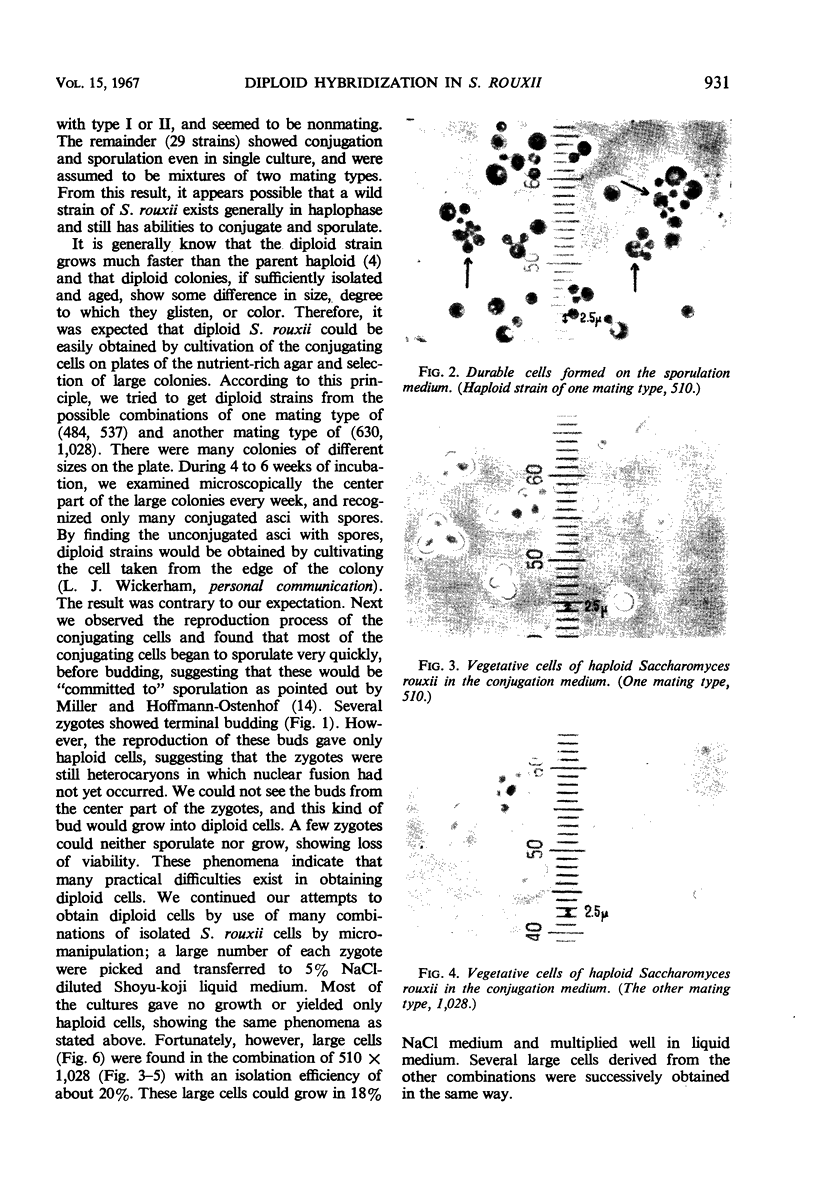
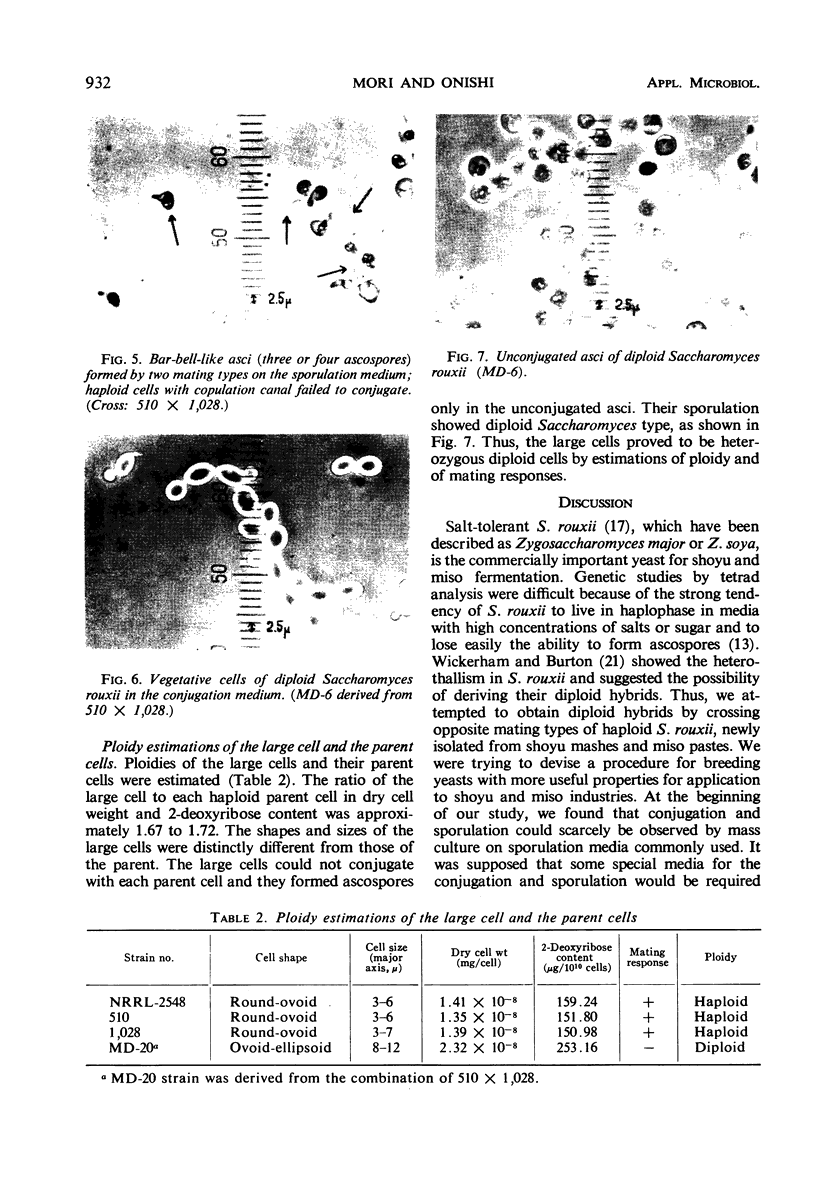
By crossing of a heterothallic haploid yeast, Saccharomyces rouxii, we have succeeded in obtaining diploid hybrids. This paper shows one possible method of breeding heterothallic haploid yeasts for industrial application. S. rouxii is highly salt-tolerant and plays an important role in shoyu and miso fermentation. Therefore, genetic improvements of the properties are of commercial importance. Since newly isolated S. rouxii could neither conjugate nor sporulate on sporulation media commonly used, a suitable medium for conjugation and sporulation of S. rouxii was firstly investigated. A 5% NaCl Shoyu-koji extract agar was found to be most efficient. Next, we tried to get diploid strains by mass culture of two mating types on the conjugation medium, but several phenomena made this difficult: (i) zygotes quickly sporulated before budding; (ii) several zygotes showed terminal budding, but the buds could not grow into diploid cells, suggesting they would be heterocaryon; and (iii) a few zygotes lost their viability. After trying to isolate and cultivate a large number of zygotes in various combinations of crossing by micromanipulation, we fortunately recognized that large cells arose from some combinations. The analysis of ploidy suggested that the large cells would be diploid. Also, they showed sporulation of typical Saccharomyces, i.e., two to four spores in an unconjugated ascus. The diploid strains thus obtained were highly salt-tolerant and stable in liquid medium. Therefore, the procedure presented here would be effective for breeding salt-tolerant S. rouxii.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCK T. D. Physiology of the conjugation process in the yeast Hansenula wingei. J Gen Microbiol. 1961 Nov;26:487–497. doi: 10.1099/00221287-26-3-487. [DOI] [PubMed] [Google Scholar]
- CERIOTTI G. A microchemical determination of desoxyribonucleic acid. J Biol Chem. 1952 Sep;198(1):297–303. [PubMed] [Google Scholar]
- CERIOTTI G. Determination of nucleic acids in animal tissues. J Biol Chem. 1955 May;214(1):59–70. [PubMed] [Google Scholar]
- FOWELL R. R. Sodium acetate agar as a sporulation medium for yeast. Nature. 1952 Oct 4;170(4327):578–578. doi: 10.1038/170578a0. [DOI] [PubMed] [Google Scholar]
- MILLER J. J., HOFFMANN-OSTENHOF C. SPORE FORMATION AND GERMINATION IN SACCHAROMYCES. Z Allg Mikrobiol. 1964;4:273–294. doi: 10.1002/jobm.3630040404. [DOI] [PubMed] [Google Scholar]
- ONISHI H. OSMOPHILIC YEASTS. Adv Food Res. 1963;12:53–94. [PubMed] [Google Scholar]
- WICKERHAM L. J., BURTON K. A. Heterothallism in Saccharomyces rouxii. J Bacteriol. 1960 Oct;80:492–495. doi: 10.1128/jb.80.4.492-495.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]