Abstract
Mucolipidosis II and III alpha/beta (ML II/III alpha/beta) are rare autosomal recessive lysosomal storage diseases that are caused by a deficiency of UDP-GlcNAc:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase, the enzyme responsible for the synthesis of the mannose 6-phosphate targeting signal on lysosomal hydrolases. A Brazilian patient suspected of having a very mild ML III was investigated using whole next-generation sequencing (NGS). Two mutations in the GNPTAB gene were detected and confirmed to be in trans status by parental analysis: c.1208T>C (p.Ile403Thr), previously reported as being pathogenic, and the novel mutation c.1723G>A (p.Gly575Arg). This study demonstrates the effectiveness of using whole NGS for the molecular diagnosis of very mild ML III alpha/beta patients.
Keywords: Mucolipidosis II/III alpha/beta, Whole exome sequencing, Molecular diagnosis
1. Introduction
In recent years, the use of massive parallel sequencing, or next-generation sequencing (NGS), is revolutionizing genetic investigation as well as clinical practice, mostly when dealing with rare diseases. It is particularly useful when the same phenotype can be caused by mutations in different genes. As cost of sequencing is progressively coming down, it is expected that in the near future comprehensive molecular diagnosis will become a standard of care. NGS can be used in several ways: as a panel targeting selected genes, as a test where the whole exome (i.e., the ~ 2% coding regions of the genome) is captured and sequenced, or as an exam where the whole genome, with its coding and non-coding regions, is sequenced. Even though sequencing a limited number of genes is apparently more rationale than all genes, developing several panels can be expensive and burdensome. Additionally, having a single test (as whole exome or genome sequencing) for thousands of different genetic disorders is practical and gives opportunities for new discoveries.
Mucolipidosis II and III (ML II and III) are autosomal recessive lysosomal disorders (LSD) in which the essential mannose 6-phosphate (Man-6-P) recognition marker system is deficient. ML II and III are caused by mutations in the GNPTAB or GNPTG genes, which encode the subunits α and β (GNPTAB) or γ (GNPTG) of the N-acetylglucosamine-1-phosphotransferase (phosphotransferase, EC 2.7.8.17). Phosphotransferase is a hexameric enzyme (2 α, 2 β, and 2 γ subunits) that mediates the first step of the synthesis of Man-6-P, but there are other genes involved in this pathway, such as the NAGPA gene which encodes “uncovering enzyme” (EC 3.1.4.45), (reviewed in [1]) and the MBTPS gene that encodes the S1P enzyme. S1P or “site-1 protease” (EC 3.4.21.112) acts post-translationally to cleave the inactive α/β precursor to generate the α and β subunits of mature phosphotransferase [2]. At least theoretically, allelic mutations in the NAGPA or MBTPS genes could cause ML II/III, even though they have never been reported.
ML is classified as II or III according to the clinical manifestations. Although there is a phenotypic spectrum, ML II (MIM# 252500) is more severe, clinically evident at birth, and usually fatal during childhood. ML III (MIM# 252600 and 252605) has a later onset of symptoms and slower progression. Clinically, skeletal alterations similar to dysostosis multiplex, claw hands, failure to thrive, and coarse facial features are observed. Milder cases may have a normal or near normal survival and absence of cognitive involvement, but still present bone involvement and claw hands. Patients with ML II and most patients with ML III have been found to be homozygous or compound heterozygous for mutations in GNPTAB and, consequently, show alterations in subunits alpha and beta (being called ML II or III alpha/beta patients). In addition, a second group of ML III patients is homozygous or compound heterozygous for mutations in GNPTG, present abnormal gamma subunit (thus being ML III gamma patients) (reviewed in [3]). As the catalytic domain of phosphotransferase is located in the α/β region, patients with ML III gamma are believed to present the milder phenotypes. However, very few patients are described with ML III gamma, which prevent us from making any kind of generalization.
Schrader et al. [4] were the first to demonstrate the effectiveness of molecular diagnosis for ML III using NGS. They were able to show a 6-bp deletion in the GNPTG gene in a family with retinitis pigmentosa and skeletal abnormalities, patients who were not previously known to have ML III. In turn, using targeted NGS, Yang et al. [5] identified two homozygous nonsense mutations in the GNPTAB gene in two Chinese families, patients who had previously been diagnosed through biochemical assays as having ML II.
In this work, we evaluated the reliability and feasibility of molecular diagnosis by whole exome sequencing for ML II/III.
2. Materials and methods
2.1. Cation-independent Man-6-P receptor (CI-MPR) affinity chromatography
25 μL of the patient's plasma or 200 μL plasma from 2 healthy individuals was diluted in 50 mM imidazole, 150 mM NaCl, 0.05% Triton X-100, pH = 6.5 (Buffer A) in a total volume of 1 mL and loaded on a CI-MPR affinity column [6]. The column was washed with 7 mL buffer A, 10 mL buffer A containing 5 mM glucose 6-phosphate and eluted with 5 mL buffer A containing 10 mM Man-6-P. The lysosomal enzyme activities were measured in the plasma and the different fractions as described [6]. All enzymatic reactions were performed in 50 mM citrate buffer containing 0.5% Triton X-100, pH = 4.6.
2.2. Whole next-generation sequencing
Peripheral blood was extracted using Easy DNA kit (Invitrogen). Whole genome sequencing was performed with Nextera Exome Capture System, followed by NGS with Illumina HiSeq 2500 (Mendelics Genomic Analysis). The study was approved by the Hospital de Clínicas de Porto Alegre (HCPA) Research Ethics Committee.
3. Results
3.1. Case report
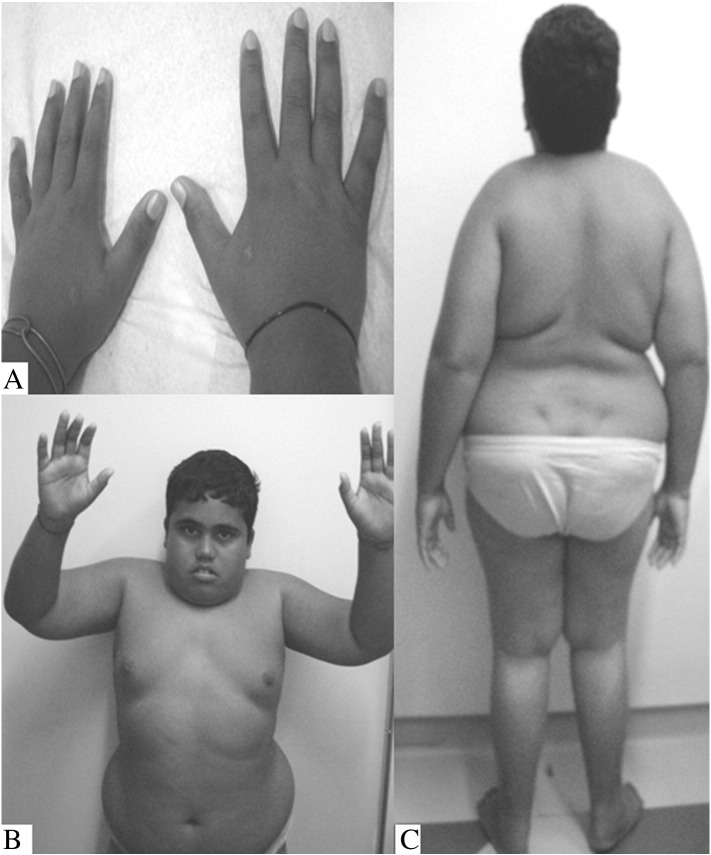
The first child born to a non-consanguineous couple, this male patient had normal development (e.g., walked at 10 months). At the age of 4, claudication was first noted. There was no history of respiratory infections or other significant comorbidity. Since the age of 8, his weight had been p > 95, and his height, between p10 and 25 for his age. At the age of 11, after being followed by an orthopedics service, he was referred to our medical genetics service for suspected multiple epiphyseal dysplasia. His skeletal radiographs, however, were suggestive of a lysosomal disorder (e.g., dysostosis multiplex was present) and showed the following: a decrease in intervertebral spaces especially in the thoracolumbar transition, a decrease in the T10 and T11 vertebrae, oval-shaped L1/L2 vertebrae, flattening of the head of the humerus with an increase in the humerus-acromion distance, as well as bilateral alterations in the femoral head suggestive of Legg–Calvé–Perthes disease. His echocardiogram, complete ophthalmologic exam, and abdominal echography were normal, as well as serum levels of calcium (8.9 mg/dL; NRV = 8.9–10.7) and alkaline phosphatase (290 U/L; NRV = 98–317). On physical exam, he presented normal head circumference, atypical facies, varus knees, restricted range of motion of the shoulders, wrists, and knees and absence of corneal clouding, claw hands, and hepatosplenomegaly (Fig. 1). At the age of 14, his height was 149 cm (p < 5) and at 18, 152 cm (p < 5). He attended regular school and did not exhibit cognitive delay.
Fig. 1.
Male patient with mucolipidosis type III, aged 14 years old, presenting a milder phenotype. A — Absence of hand joint contractures. B — Joint contractures in shoulders, hindering arm elevation above the head; atypical facies. C — Whole body posterior image. The obesity is evident.
3.2. Biochemical analysis
Based on the clinical findings, the initial diagnosis raised was mucopolysaccharidosis type IV A or Morquio A syndrome (MPS IV-A, MIM# 253000), which was ruled out due to the normal activity of N-acetylgalactosamine-6-sulfatase (EC 3.1.6.4) in leukocytes (Table 1). As the patient exhibited dysostosis multiplex by X-ray, a biochemical investigation for lysosomal storage disorders was requested, which was suggestive of ML II/III (presence of an increased activity of lysosomal hydrolases in plasma and normal activity in leukocytes). Since there were no fibroblasts available for biochemical analysis, the presence of Man-6-P-containing lysosomal acid hydrolases in the plasma was investigated using CI-MPR affinity chromatography. As shown in Table 2, this analysis showed a striking decrease in phosphorylated acid hydrolases in the patient's plasma compared to normal, consistent with the diagnosis of ML II/III.
Table 1.
Biochemical characterization of patient.
| Biochemical investigation | Sample | Patient | Reference Values |
|---|---|---|---|
| α-l-Iduronidase (EC 3.2.1.76) | Plasma | 119 | 6.8–13.7 |
| β-Glucuronidase (EC 3.2.1.31) | Plasma | 906 | 30–300 |
| α-N-acetylglucosaminidase (EC 3.2.1.50) | Plasma | 875 | 34–162 |
| α-Mannosidase (EC 3.2.1.24) | Plasma | 2026 | 17–56 |
| β-Hexosaminidases A (EC 3.2.1.30) | Plasma | 11,747 | 550–1675 |
| β-Hexosaminidases B (EC 3.2.1.30) | Plasma | 27,535 | 265–1219 |
| β-Hexosaminidases, total (EC 3.2.1.30) | Plasma | 39,282 | 1000–2857 |
| Chitotriosidase (EC 3.2.1.14) | Plasma | 98 | 8.8–132 |
| Iduronate 2-sulfatase (EC 3.1.6.12) | Plasma | 1716 | 122–463 |
| β-Galactosidase (EC 3.2.1.23) | Leukocyte | 162 | 78–280 |
| α-l-Iduronidase (EC 3.2.1.76) | Leukocyte | 72 | 32–56 |
| Arylsulfatase B (EC 3.1.6.12) | Leukocyte | – | 72–176 |
| β-Glucosidase (EC 3.2.1.21) | Leukocyte | 19 | 10–45 |
| α-N-acetylglucosaminidase (EC 3.2.1.50) | Leukocyte | 64 | 68–352 |
| Iduronate 2-sulfatase (EC 3.1.6.12) | Leukocyte | 95 | 31–110 |
| β-Glucuronidase (EC 3.2.1.31) | Leukocyte | 175 | 23–151 |
| Sphingomyelinase (EC 3.1.4.12) | Leukocyte | 1.9 | 0.74–4.9 |
| Total β-hexosaminidases (EC 3.2.1.30) | Leukocyte | 7384 | 552–16,662 |
| β-Hexosaminidase A | Leukocyte | 53 | 150–390 |
| N-acetylgalactosamine-6-sulfatase | Leukocyte | 29 | 14–81 |
| Dosage of GAGs | Urine | 128 (79–256) | < 9 years: 44–106 mg/L |
| Thin-layer chromatography of GAGs | Urine | DS + HS + CS/HS | Normal |
| Thin-layer chromatography of sialoligosaccharide | Urine | Normal | Normal |
GAGs: Glycosaminoglycans; –: not available; DS: dermatan sulfate; HS: heparan sulfate; CS: chondroitin sulfate.
Table 2.
CI-MPR affinity chromatography: presence of phosphorylated acid hydrolases in plasma.
| % enzyme bound to CI-MPR column | |||
|---|---|---|---|
| Enzyme | Control 1 | Control 2 | Patient |
| α-Mannosidase | 12.5 | 11.2 | 0.5 |
| β-Glucuronidase | 5.9 | 14.7 | 0.1 |
| β-Hexosaminidase | 7.7 | 14.3 | 0 |
| β-Mannosidase | 13.5 | 19.7 | 0.4 |
Although the investigation performed in this patient is considered enough for confirming ML II/III, due to the very mild clinical picture, especially the absence of claw hands – which is an unexpected finding even for ML III gamma – it was decided that whole NGS would be performed, also aiming to exclude the presence of pathogenic mutation in other genes involved in M6P biosynthesis (such as NAGPA and MBTPS).
3.3. Whole next-generation sequencing
Whole NGS generated 64,363,166 sequences, each target base was read on average 92 × and 94% of the target bases were read at least 10 ×. Two heterozygous variants in GNPTAB were identified: c.1208T>C (p.Ile403Thr), previously reported as deleterious [7], and the novel variant c.1723G>A (p.Gly575Arg), which was not present in more than 8000 normal controls (Exome Server Variant and 1000 Genomes), including 1000 Brazilians (processed as controls at the NGS laboratory). No pathogenic variants were found in the GNPTG, NAGPA, and MBTPS genes. This result was confirmed by Sanger sequencing, and a segregation study demonstrated that the father was a carrier of the variant p.Ile403Thr, and the mother, of the transversion p.Gly575Arg.
Polyphen-2 predicted p.Gly575Arg as probably damaging (score of 0.974). Sift v.1.1.3 prediction indicated the variant p.Gly575Arg as tolerated/neutral. The MutPred software interpreted the pathogenicity as an actionable hypothesis, with a probability score of deleterious mutation of 0.382, supposedly causing the following: gain of solvent accessibility (p = 0.0171), gain of helix (p = 0.0425), gain of relative solvent accessibility (p = 0.0479), loss of loop (p = 0.0512), and loss of methylation at K573 (p = 0.0536).
4. Discussion
The aim of this work was to evaluate the molecular diagnosis of very mild ML III using whole NGS, and our results demonstrate the utility of this new technology.
The confirmation of the clinical diagnosis of ML II/III faces several challenges in regard to the performance of biochemical assays, since these assays are not widely available (nor easily done) and depend on the performance of fibroblast biopsy. The phosphotransferase assay, for instance, requires a radioactive substrate, is difficult to implement, and few groups in the world perform this assay.
Currently, biochemical diagnosis of ML II/III is usually performed indirectly, through the measurement of lysosomal hydrolases both in plasma (their activity should be high) and in fibroblasts (their activity should be low). When fibroblasts are not available, other tests are needed (such as the analysis of phosphorylated residues, which is also performed on a research basis, or DNA analysis only). Moreover, as occurred in the present case, the clinical picture can be so mild, sometimes limited to bone disease, that even experienced physicians miss its diagnosis.
Regarding DNA analysis, there is no consensus about which gene (GNPTAB or GNPTG) should be first analyzed when facing a case with clinical picture compatible with ML III. If the case is very mild, an initial analysis of GNPTG may be suggested, but, as reported herein, mutations in GNPTAB might also cause very mild phenotypes. Among the available technologies for DNA analysis, exome sequencing is one of the most modern tools available. It is important to point out that ML III presents locus heterogeneity and that Sanger sequencing of whole coding GNPTG and GNPTAB is made by amplification and sequencing of the 11 and 21 exons of each gene, respectively, a situation that requires time and investment and can delay the diagnosis. In this regard, exome sequencing is a reliable alternative for confirmation of the diagnosis of ML III.
However, different sequencing platforms vary in their ability to identify variants, even when sequencing the same genome. Besides that, prediction of the pathogenicity of the novel missense variants found is still a problem. For instance, the patient reported herein is a compound heterozygote for two missense mutations in the GNPTAB gene, p.Ile403Thr (a previously reported pathogenic mutation) and p.Gly575Arg (a novel mutation which presents divergent results regarding its pathogenicity by bioinformatics analysis). Up to date, more than 125 different mutations of GNPTAB, encoding the α/β-subunit precursor of the phosphotransferase, have been described to cause ML II or III alpha/beta (HGMD, 2014). In general ML II alpha/beta patients have nonsense, frameshift or splice-site mutations in GNPTAB, whereas ML III alpha/beta patients carry missense mutations [8].
Franke, Braulke, & Storch [9] showed that for efficient transport of the α/β-subunit precursor protein from the ER to the Golgi apparatus, a nonexchangeable dileucine (Leu 5-Leu 6) and the dibasic motif (Arg1253-Ile1254-Arg1255) are required in its cytosolic N- and C-terminal domains, respectively. After, the S1P-mediated cleavage α/β-subunit precursor protein occurs in the Golgi apparatus, a prerequisite for the catalytic activity of phosphotransferase [10].
The mature human α-subunit is a type II membrane protein with an N-terminal tail, a transmembrane region and a subsequent luminal domain, each one comprising 19, 22, and 886 amino acids respectively [11], [12]. The luminal domain contains 17 potential N-glycosylation sites, and mutations associated to ML II and ML III are described in this domain [12], [13], [14].
The missense mutation p.Gly575Arg changes a polar amino acid (glycine, GGA) for an apolar (Arginine, AGA). It is located in the luminal domain of the α-subunit, in a region with no homolog domains, but next to an N-glycosylation site (N580). This residue and amino acid (Gly575) is highly conserved in mammals, fish, amphibians, birds, but not in zebrafish and frogs, and might be a binding site for luminal ER proteins required for ER exit of α/β-subunit precursor. Alternatively, p.Gly575Arg can cause misfolding of the mutant protein and retention in the ER [15]. Both alternatives could explain the pathogenicity of p.Gly575Arg and the patient's mild phenotype. There is no other mutation described in the 575 residue of phosphotransferase — the closest one is p.Arg587Pro (c.1760G>C) which is also associated to ML III [16].
The following evidence also suggests p.Gly575Arg is pathogenic: 1) p.Ile403Thr is described in ML III patients [7], [8], [17] and is presumed to be a mild mutation by expression studies [7]. The very mild phenotype of the patient could be explained by the fact he is compound heterozygous for two mild mutations (p.Ile403Thr and p.Gly575Arg); 2) no other mutations causing the phenotype were found in the GNPTAB, GNPTG, NAGPA, and MBTPS genes; 3) both mutations found are in trans and inherited; 4) p.Gly575Arg was not found in a high number of controls from the same population as the patient.
We agree with Schrader et al. [4] and reinforce the idea that the costs of NGS will gradually come down. As soon as this happens, this technique will become the most direct approach for the diagnosis of Mendelian disorders that are phenotypically and genetically heterogeneous, such as ML II/III. In the meantime, Sanger sequencing of GNPTAB and GNPTG remains the first strategy for DNA analysis of patients with ML II/III.
Acknowledgments
We thank the family who participated in this study for their outstanding collaboration. This project was supported by FIPE–HCPA, FAPERGS, CAPES, CNPq (Brazil), the Postgraduate Program in Genetics and Molecular Biology of UFRGS, and National Institutes of Health grant CA-008759.
References
- 1.Coutinho M.F. Handbook of Glycosyltransferases and Related Genes. 2014. N-acetylglucosamine-1-phosphate transferase, alpha/beta and gamma subunits (GNPTAB, GNPTG) pp. 1335–1347. [Google Scholar]
- 2.Marschner K., Kollmann K., Schweizer M. A key enzyme in the biogenesis of lysosomes is a protease that regulates cholesterol metabolism. Science. 2011;333:87–90. doi: 10.1126/science.1205677. [DOI] [PubMed] [Google Scholar]
- 3.Cathey S.S., Leroy J.G., Wood T., Eaves K. Phenotype and genotype in mucolipidosis II and III alpha/beta: a study of 61 probands. J. Med. Genet. 2010;47:38–48. doi: 10.1136/jmg.2009.067736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrader K.A., Heravi-Moussavi A., Waterset P.J. Using next-generation sequencing for the diagnosis of rare disorders: a family with retinitis pigmentosa and skeletal abnormalities. J. Pathol. 2011;225:12–18. doi: 10.1002/path.2941. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y., Wu J., Liu H. Two homozygous nonsense mutations of GNPTAB gene in two Chinese families with mucolipidosis II alpha/beta using targeted next-generation sequencing. Genomics. 2013;102:169–173. doi: 10.1016/j.ygeno.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Vogel P., Payne B.J., Read R. Comparative pathology of murine mucolipidosis types II and IIIC. Vet. Pathol. 2009;46:313–324. doi: 10.1354/vp.46-2-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tappino B., Chuzhanova N.A., Regis S. Molecular characterization of 22 novel UDP N acetylglucosamine 1 phosphate transferase α and β subunit (GNPTAB) gene mutations causing mucolipidosis types IIα/β and IIIα/β in 46 patients. Hum. Mutat. 2009;30(11):E956–E973. doi: 10.1002/humu.21099. [DOI] [PubMed] [Google Scholar]
- 8.Encarnação M. Molecular analysis of the GNPTAB and GNPTG genes in 13 patients with mucolipidosis type II or type III-identification of eight novel mutations. Clin. Genet. 2009;76(1):76–84. doi: 10.1111/j.1399-0004.2009.01185.x. [DOI] [PubMed] [Google Scholar]
- 9.Franke M., Braulke T., Storch S. Transport of the GlcNAc-1-phosphotransferase α/β-subunit precursor protein to the Golgi apparatus requires a combinatorial sorting motif. J. Biol. Chem. 2013;288:1238–1249. doi: 10.1074/jbc.M112.407676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudo M., Brem M.S., Canfield W.M. Mucolipidosis II (I-cell disease) and mucolipidosis IIIA (classical pseudo-Hurler polydystrophy) are caused by mutations in the GlcNAc-phosphotransferase α/β–subunits precursor gene. Am. J. Hum. Genet. 2006;78(3):451–463. doi: 10.1086/500849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudo M., Bao M., D'Souza A., Ying F., Pan H., Roe B. a, Canfield W.M. The alpha- and beta-subunits of the human UDP-N-acetylglucosamine:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase [corrected] are encoded by a single cDNA. J. Biol. Chem. 2005;280:36141–36149. doi: 10.1074/jbc.M509008200. [DOI] [PubMed] [Google Scholar]
- 12.Tiede Stephan. Mucolipidosis II is caused by mutations in GNPTA encoding the α/β GlcNAc-1-phosphotransferase. Nat. Med. 2005;11(10):1109–1112. doi: 10.1038/nm1305. [DOI] [PubMed] [Google Scholar]
- 13.Kollmann K. Mannose phosphorylation in health and disease. Eur. J. Cell Biol. 2010;89(1):117–123. doi: 10.1016/j.ejcb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Lupas M., Van Dyke J.S. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 15.Pace R. Mucolipidosis II‐related mutations inhibit the exit from the endoplasmic Reticulum and proteolytic cleavage of GlcNAc‐1‐phosphotransferase precursor protein (GNPTAB) Hum. Mutat. 2014;35(3):368–376. doi: 10.1002/humu.22502. [DOI] [PubMed] [Google Scholar]
- 16.Lam C.W., Yan M.S.C., Li C.K., Lau K.C., Tong S.F., Tang H.Y. DNA-based diagnosis of mucolipidosis type IIIA and mucopolysacchariodisis type VI in a Chinese family: a chance of 1 in 7.6 trillion. Clin. Chim. Acta. 2007;376:250–252. doi: 10.1016/j.cca.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Cury G.K. Mucolipidosis II and III alpha/beta in Brazil: analysis of the GNPTAB gene. Gene. 2013;524(1):59–64. doi: 10.1016/j.gene.2013.03.105. [DOI] [PubMed] [Google Scholar]