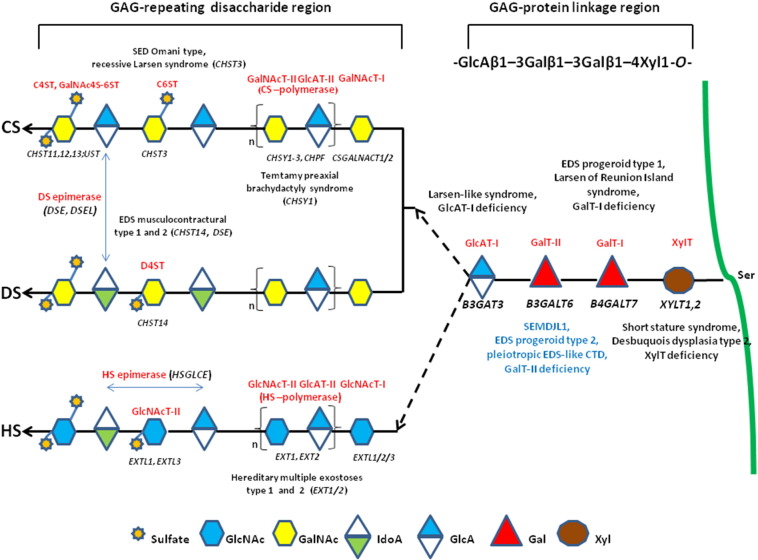
Fig. 1.
Schematic presentation of the synthesis of the GAG backbones of CS/DS or HS chains and related genetic disorders. Each enzyme (glycosyltransferase and/or epimerase, in red) and its coding gene (in black) are described near the sugar symbols. After the synthesis of specific core proteins, the synthesis of the GAG-protein linkage region is initiated by XylT, which transfers [Xyl] to the specific Ser in the endoplasmic reticulum. The synthesis of the linkage region is completed by the consecutive addition of two molecules of [Gal], added by GalT-I/II, followed by the transfer of [GlcA] catalyzed by GlcAT-I in the Golgi. The first [GalNAc] is transferred to the [GlcA] in the linkage region by GalNAcT-I, resulting in the initiation of the synthesis of the repeating disaccharide region of CS/DS chains. Alternatively, the addition of the [GlcNAc] to the linkage region by GlcNAcT-I induces HS biosynthesis. The repeating disaccharide region of the CS/DS chain is elongated by alternate additions of [GlcA] and [GalNAc] catalyzed by CS-GlcAT-II and GalNAcT-II activities of a heterocomplex formed by ChSy and ChPF. The polymerization of the HS chain is catalyzed by HS-GlcAT-II and GlcNAcT-II activities. GAG chains are finally matured by tightly controlled modifications, i.e., epimerization and sulfation. Concerning CS chains, sulfation occurs mainly at positions 4 and 6 of [GalNAc] and position 2 of [GlcA] and is catalyzed by various sulfotransferases. After the formation of the chondroitin backbone, [GlcA] is converted into [IdoA] resulting in the formation of the dermatan backbone, in which position 4 of [GalNAc] is sulfated by D4ST. The unbranched HS chain undergoes final maturation, which consists of a complex series of processing reactions involving [GlcNAc] deacetylation and sulfation, epimerization of [GlcA], and subsequent O-sulfation at different positions (for an extensive review see reference [6]).