Figure 3.
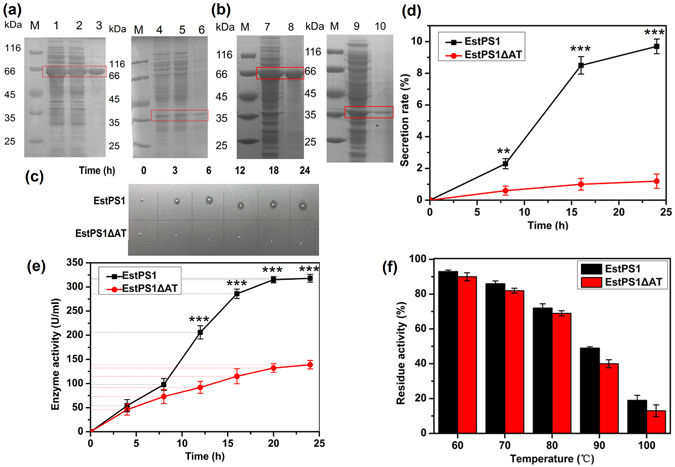
Role of the AT domain in recombinant EstPS1. (a) SDS-PAGE analysis of EstPS1 and EstPS1ΔAT. lane M - standard marker proteins; lane 1,2 - supernatant of cell lysate (EstPS1); lane 3 - purified protein of EstPS1; lane 4,5 - supernatant of cell lysate (EstPS1ΔAT); lane 6 - purified protein of EstPS1ΔAT. (b) SDS-PAGE analysis of EstPS1ΔSP and EstPS1ΔSP + AT. lane M - standard marker proteins; lane 7 - crude extracts of EstPS1ΔSP; lane 8 - purified protein of EstPS1ΔSP (inclusion bodies); lane 9 - crude extracts of EstPS1ΔSP + AT; lane 10 - purified protein of EstPS1ΔSP + AT (inclusion bodies). (c) Transparent zones of EstPS1 and EstPS1ΔAT in the solid induction medium at different hours. (d) Effect of the AT domain on secretion rate at 0 h, 8 h, 16 h, and 24 h. Secretion rate is defined as the enzyme activity of culture medium in proportion to the total activity. Data represent mean ± SD (n = 3); *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s-t test. (e) Comparison of the total activity (fermentation broth) between EstPS1 and EstPS1ΔAT with respect to the AT domain. (f) Residual activities of the enzymes after incubation at various temperatures (60–100 °C) for 10 min.
