Figure 1.
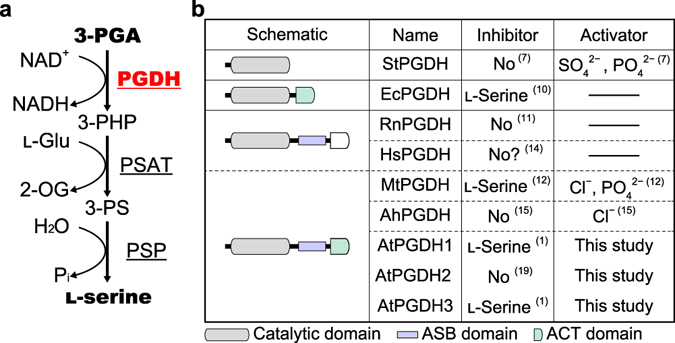
Enzymatic functions, domain architectures, and effectors of 3-phosphoglycerate dehydrogenase. (a) Schematic of the phosphorylated pathway; names of enzymes are underlined; PGDH, 3-phosphoglycerate dehydrogenase; PSAT, phosphoserine aminotransferase; PSP, phosphoserine phosphatase; 3-PHP, 3-phosphohydroxypyruvate; 3-PS, 3-phosphoserine; l-Glu, l-glutamate; 2-OG, 2-oxoglutarate; Pi, inorganic phosphate. (b) Domain architectures and effectors of PGDH; the domains of PGDH proteins from archaea [S. tokodaii (StPGDH)], bacteria [E. coli (EcPGDH) and M. tuberculosis (MtPGDH)], cyanobacterium [A. halophytica (AhPGDH)], mammals [R. norvegicus (RnPGDH) and Homo sapiens (HsPGDH)], and plant [A. thaliana (AtPGDHs)] are schematically shown with highlighted catalytic, ACT, and ASB domains. Because ACT domains of HsPGDH and RnPGDH are not functional, they are indicated in white. Numbers in parenthesis indicate references. In ref. 14, HsPGDH was not inhibited by serine, although the data were not shown (“No ? ” in the figure).
