Figure 4.
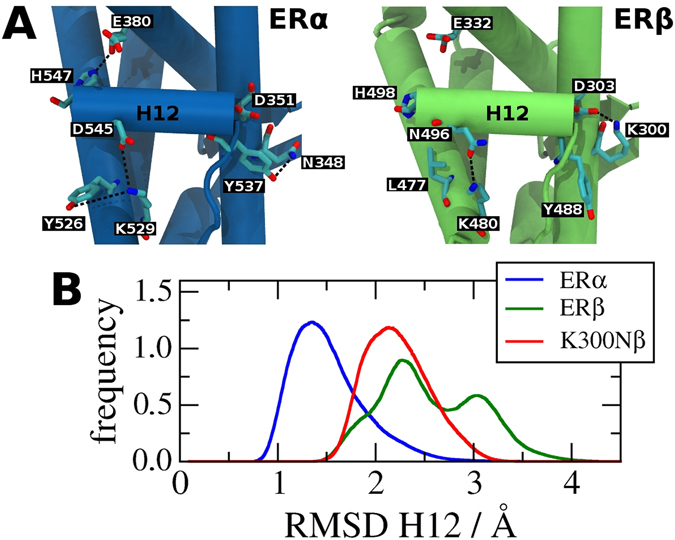
Stability of H12 canonical position in ER subtypes: (A) Comparison of main interactions between H12 and the remaining canonical LBD structures of the ERα LBD-E2 (blue, PDB code 1QKU) and ERβ LBD-E2 (green, PDB code 3OLS). ERα LBD-E2 has three hydrophilic interactions stabilizing H12 in its canonical position while ERβ LBD-E2 has only one interaction. The position of histidine H498 was not obtained in 3OLS structure. Its position was added based on the structure of the phosphorylated ERβ LBD-E2 (PDB code 3OLL). (B) RMSD distribution of H12 computed with respect to MD simulation average structures of wild-type ERα LBD-E2 (blue), wild-type ERβ LBD-E2 (green) and K300N mutant of ERβ LBD-E2 (K300Nβ). H12 of wild-type ERα shows a lower mobility than wild-type ERβ. K300Nβ corresponds to the transformation β → α of one of the residues that stabilizes H12. As expected, this mutation reduces the mobility of this helix.
