FIGURE 3.
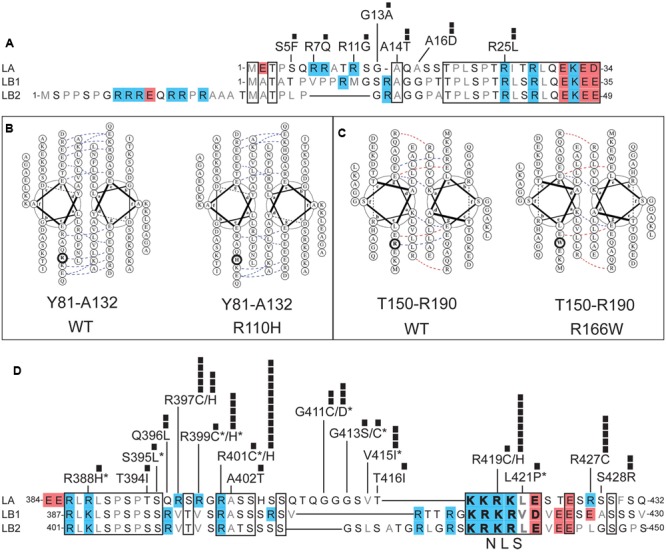
Positions and potential consequences of novel variants in the head, coiled-coil rod or neck domains. (A) Head domain amino acid sequences of human lamin A (LA; UniProt P02545), lamin B1 (LB1; UniProt P20700) and lamin B2 (LB2; UniProt Q03252). Regions conserved among LA, LB1, and LB2 are boxed. Polar residues are lettered in black; hydrophobic residues in gray. Each black square represents one individual with the indicated variant. (B,C) Loss of coiled-coil backbone associations between parallel rod domains predicted for: (B) novel variant p.R110H in the context of wild-type (‘WT’) residues Y81-A132, and (C) novel variant p.R166W in the context of wild-type residues T150-R190. Dotted lines indicate ionic interactions between two residues, as predicted by DrawCoil 1.0 (http://www.grigoryanlab.org/drawcoil/). (D) Amino acid sequences of the neck domains of human lamin A (residues 384–432), lamin B1 (residues 387–430) and lamin B2 (residues 401–450). Nuclear localization signal (NLS) residues are bolded. Specific ExAC variants, and the number of individuals (black squares) with each variant, are shown. Asterisks indicate ExAC variants also reported in the laminopathy database.
