Figure 2. RKIP induces positive inotropy and protects from cell death and diastolic Ca2+ leak.
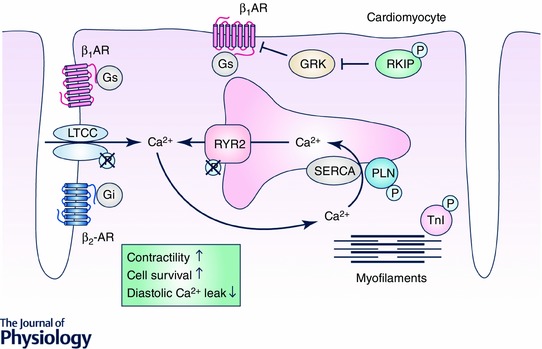
The Raf kinase inhibitor protein (RKIP) binds GRK2 and inhibits G‐protein‐coupled receptor kinase (GRK)‐mediated receptor phosphorylation, which prevents receptor desensitization and internalization and, thus, increases β‐adrenergic receptor signalling. RKIP increases contractility and relaxation of cardiomyocytes via activated β1‐adrenergic receptor (β1AR) coupled to stimulatory G‐proteins (Gs): phosphorylated phospholamban (PLN) dissociates from sarco‐/endoplasmatic reticulum Ca2+‐ATPase (SERCA2a) and thereby increases SERCA2a activity, Ca2+ loading of the sarcoplasmatic reticulum and cardiomyocyte contractility. Phosphorylation of troponin I (TnI) decreases Ca2+ sensivity and thereby increases cardiomyocyte relaxation. RKIP mediates anti‐apoptotic, anti‐fibrotic and anti‐arrhythmic effects via increased β2‐adrenergic receptor (β2AR) signalling. Continuous signalling of β2AR coupled to inhibitory G‐proteins (Gi) prevents β1AR‐stimulated increases in ryanodine receptor 2 (RyR2) and L‐type Ca2+ channel (LTCC) phosphorylation and protects from diastolic Ca2+ leak.
