Abstract
Background:
Mammary hyperplasia is one of the most common benign breast disorders. Although traditional Chinese medicine has a vast experience in the treatment of mammary hyperplasia, it is not accepted widely due to its unclear mechanism.
Methods and Materials:
To address the mechanism, we developed a mouse model of mammary hyperplasia. We gave mice estradiol valerate tablets and progesterone capsules sequentially for one month by intragastric administration.
Results:
Mice treated by this method had a series of pathological changes which are similar to those detected in women with mammary hyperplasia, including ectopic level of estradiol and progesterone in serum, hyperplasia of mammary glands and increased expression of ERα and PR.
Conclusion:
This model will facilitate the mechanical study of traditional medicine on mammary hyperplasia.
Keywords: mouse model, mammary hyperplasia, estradiol valerate, progesterone, intragastric administration
Introduction
Mammary hyperplasia, whose prevalence increases annually, is one of the leading benign breast disorders among women of reproductive age (Ferrara, 2011). Women with mammary hyperplasia are at increased risk for developing breast cancer (Silvera et al., 2008). In some areas of China, the incidence of mammary hyperplasia is almost close to 30% (Mo, 2011), constituting a severe threat to women’s physical/mental health and life quality.
Traditional Chinese medicine has a vast experience in treating mammary hyperplasia (Hu and Li, 2015), but the underneath mechanisms are not clear yet, which limiting its application worldwide. Suitable animal models are versatile experimental system to unravel these mechanic questions.
Rodents are commonly used in the field of breast research, because the mammary gland development of these animals closely resembles that of the human breast (Hovey et al., 2004; Macias, H. and Hinck, L., 2012). A rat model of mammary hyperplasia has been reported (Chen et al., 2015), whereas how to set up a mouse model is still not clear. The rat model, in certain ways, is easier to use and is very reproducible. However, the mouse model has the advantage of manipulating genetic mutations within the mammary epithelial cell. Thus, the suspected mechanism of the traditional medicine can be effectively investigated in the mice with specific gene mutations. In addition, mice require less consumption of food and drug, and the corresponding research cost is also lower. Therefore, it is necessary to establish a mammary hyperplasia model in mice.
Estrogen and progesterone are pivotal hormones for mammary ductal elongation and branching during puberty (Arendt et al., 2015). Their concentrations and receptors are elevated in the mammary glands of patients with benign breast disease (Schillace et al., 2014). In adult mice, if the proliferation of mammary gland is arrested by ovariectomy, exogenous estrogen alone provides a weak signal, whereas treatment with estrogen and progesterone fully rescues glandular proliferation (Diep et al., 2015). Besides, intramuscular injection of estrogen and progesterone leads to mammary hyperplasia in rats (Chen et al., 2015). Based on these facts, we plan to induce mammary hyperplasia in mice by giving extra estrogen and progesterone.
As mice are smaller than rats, intramuscular injection is not suitable for frequent administrations. Intragastric administration may be a better option. Here, we chose estradiol valerate tablet and progesterone soft capsule, which are two common oral hormones in clinic. We treated mice sequentially with these two drugs by intragastric administration and successfully induced a hyperplasia phenotype in mammary gland. Moreover, ectopic expressions of estrogen receptor α (ERα) and progesterone receptor (PR) were detected in the glands with hyperplasia. We believe that the mouse model of mammary hyperplasia will be an excellent alternative for the study of traditional medicine.
Materials and Methods
Reagents
Estradiol valerate tablet (PROGYNOVA®, Bayer), progesterone soft capsule (URTOGESTAN®, Laboratoires Besins International), carmine (Sigma), Permount (Thermo), anti-mouse estradiol Elisa kit and anti-mouse progesterone Elisa kit (AMEKO, Shanghai Lianshuo Biological Technology Co., Ltd).
Animals
Female C57BL/c mice (age: 6weeks; weight: 15-17g) were purchased from Vital River Laboratory (Beijing, China) and housed in a SPF room with a 12h light/dark cycle at a temperature of 22-25°C and relative humidity of 40-60%. Mice were allowed access to food and water ad libitum. All procedures were approved by the Institutional Animal care and Use Committee at Liaoning University of Traditional Chinese Medicine (permission No.20150308).
Drug preparation
An estradiol suspension was made by dissolving the estradiol valerate tablets in water to a final concentration of 0.25 mg/ml estradiol, while a progesterone suspension were prepared by cutting open the progesterone soft capsules and resuspending the contents in sesame oil to a final concentration of 2mg/ml progesterone. All the suspensions were stored at 4°C, warmed up to 37°C and mixed several times upon using to treat animals.
Treatments
At age of 7 weeks, the mice were randomly divided into 2 groups of 20 mice each: vehicle-treated (control) and hormone-treated. Hormone-treated mice were orally administered estradiol (2.5 mg/kg body weight) once every two days for 12 times, followed by progesterone (20 mg/kg body weight) once a day for 5 consecutive days. The amount of drugs referenced that used in women (Ulrich et al., 1994). Vehicle-treated mice were first treated with water and subsequently with sesame oil. One hour after the last administration, 10 mice of each group were sacrificed. The rest of mice were maintained for 30 more days and then sacrificed. Blood and mammary glands were collected when the animals were sacrificed.
Mammary gland whole mount staining
Mammary glands No.4 (left inguinal) were spread on glass slides, fixed in Carnoy’s fixative overnight, washed in 70% ethanol for 30 min, changed gradually to distilled water, and stained overnight in carmine alum. Tissues were then dehydrated in a graded series of ethanol solutions, immersed in xylene until the fat was cleared off, and mounted with Permount. Images of mammary gland whole mounts were taken with a digital camera for morphological detection. Secondary and tertiary branch points within five fields per mammary gland were manually counted under the 4× objective of a microscope.
Histochemistry
Mammary glands No.4 (right inguinal) were fixed in Carnoy’s fixative, embedded in paraffin, sectioned at 6 microns. Tissue sections were processed for a standard hematoxylin-eosin staining. Signals were visualized with Olympus microscope BX41-32P02-FLB3.
Western blot
Mammary glands NO.3 were ground into a fine powder in liquid nitrogen. The total protein was extracted from the powder using the RIPA buffer (Beyotime Biotechnology) supplemented with proteinase inhibitor (Roche). Approximately 50 μg of protein per sample was separated in an 8% SDS-PAGE and transferred to a PVDF membrane. The membrane was subjected to immunoblotting with anti-ERα antibody (Cell Signaling Technology, 8644, 1:1000), anti-PR antibody (Santa Cruz Biotechnology, sc-398898, 1:1000) and anti-tubulin antibody (Vazyme Biotech). HRP conjugated secondary antibodies (Vazyme Biotech) and ECL (Beyotime Biotechnology) was used to detect the protein existence. The pictures were analyzed with Image J.
Analyses of serum estradiol and progesterone
Blood was collected by removing one eyeball. After coagulating for 90 min at room temperature, the blood was centrifuged at 2000 × g for 30 min and serum stored at −80°C until assayed. Serum level of estradiol and progesterone were measured by Elisa kits according to the supplier’s protocol. Each group contains data from 10 animals.
Statistical analysis
Data were analyzed by one-way ANOVA. Results were considered significantly different at P < 0.05. Values are expressed as mean ± standard deviation.
Results and discussion
To mimic the pathological conditions of patients with mammary hyperplasia, we treated the mice sequentially with a suspension of estradiol valerate tablets and a suspension of progesterone capsules by intragastric administration. By the time of the last administration, the serum concentration of estradiol in vehicle-treated group was 9.55±0.59 ng/L, while that in hormone-treated group was as high as 135.37±12.75 ng/L (Figure 1A). Similarly, the progesterone concentration after hormone treatment was significantly elevated from 282.80±19.06 pmol/L to 1242.6±75.02 pmol/L (Figure 1A). Although the estradiol and progesterone levels in hormone-treated group dropped dramatically within 30 days, they were still noticeably higher than those in vehicle-treated group (Figure 1A). These confirmed that intragastric administration of estradiol valerate and progesterone could increase estradiol and progesterone level abnormally in the serum of mice and this increase could be maintained for a month.
Figure 1.
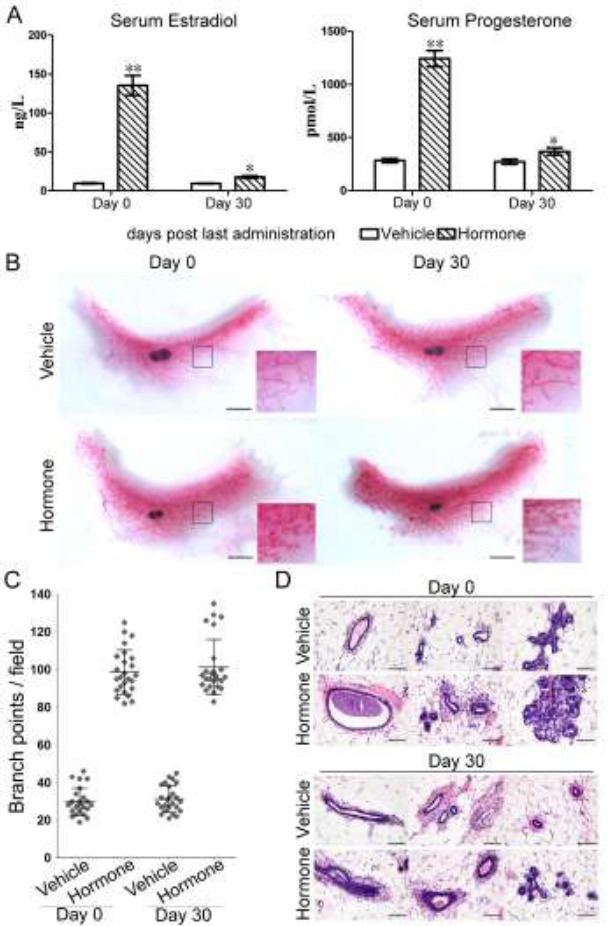
Mammary hyperplasia induced by estradiol valerate and progesterone. A. Serum levels of estradiol and progesterone were measured by Elisa on Day 0 and Day 30 after the last administration. Comparison was made between hormone-treated group and vehicle-treated group. **, p<0.001; *, p<0.05 (n=10). B. Whole mount staining was preformed to detect the morphological changes after hormone treatment. Representative samples were shown. Scale bar: 0.5 cm. C. The number of branch points was quantified under microscope. One dot represents the number of branch points in one field. (n=5). D. HE staining was applied to observe the histological changes inside the mammary gland. Scale bar: 50 micrometer.
Next, we performed whole mount staining to check if the increased level of estradiol and progesterone could affect the morphology of mammary glands. Compared with vehicle-treated glands, hormone-treated mammary glands displayed an evident hyperplasia characterized by an increased number of branches, side-branches and terminal end buds and an appearance of alveolar structures as well (Figure 1B). To quantify the morphological changes, the numbers of the secondary and tertiary branch points of mammary glands were counted under the microscope. As shown in Figure 1C, hormone treatment resulted in a notable increase in the number of branch points (p<0.05). Further, HE staining showed that hormone-treated glands had an extended lumen with more liquid, the ducts were composed of multiple layers of epithelial cells, and the alveolar buds were fully formed compared to vehicle-treated glands (Figure 1D). These morphological changes were not recovered after 30 days of hormone withdrawal (Figure 1B and 1D). Thus, intragastric administration of estradiol valerate tablets and progesterone capsules can induce mammary hyperplasia in mice.
In mammary epithelial cells, estradiol acts through it receptor ERα, and progesterone functions through PR (Arendt et al., 2015). We then checked the expression of ERα and PR to determine if they were involved in the morphological changes observed above. We found that the expression levels of ERα and PR were increased in mammary gland due to hormone treatment (Figure 2), which is in line with Gupta’s study (Gupta et al., 2015). Gupta and her colleagues have proven that the expression of ER and PR parallels the intensity of hyperplasia in benign breast diseases.
Figure 2.
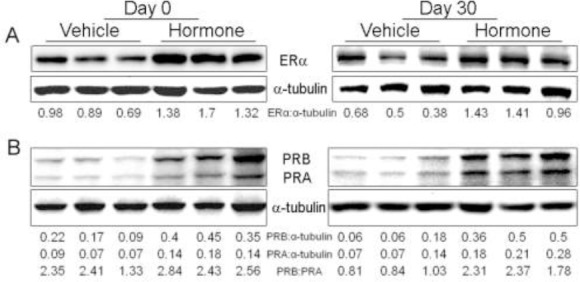
The expression of ERα and PR after hormone treatment. Protein lyses from 3 animals of each group were used to determine the expression level of ERα (A) and PR (B) by western blot. The numbers beneath western blots stand for the ratio of the intensity of target band to that of their own loading reference bands.
PR has two isoforms: PRA and PRB. PRA overexpressed in mice induces ductal hyperplasia (Shyamala et al., 1998), whereas mice lacking PRB, but not PRA, exhibit marked defects in branching and alveologenesis (Mulac-Jericevic et al., 2003), suggesting that PRB is the predominant isoform required for mammary gland development and expansion. We detected that both PRA and PRB were weakly expressed in vehicle-treated gland at the similar level (Figure 2B). They were upregulated after hormone treatment and the ratio of PRB: PRA was also increased (Figure 2B). The imbalanced PRA and PRB expression is linked to high risk of breast cancer in women (Mote et al., 2002). Therefore, the mammary hyperplasia induced by estradiol valerate and progesterone shows the similar pattern of ER and PR to that detected in women with breast hyperplasia.
In conclusion, we successfully established a mammary hyperplasia model in mice by intragastric administration of estradiol valerate and progesterone. This model will facilitate research pertaining to the mechanism study of traditional medicine.
Acknowledgements
The authors declare no competing interests, and thank Shenyang Science and Technology Plan (F14-199-4-00), The Education Department of Liaoning Province (L2013369) and National Natural Science Foundation of China (81403396) for funding.
References
- 1.Arendt LM, Kuperwasser C. Form and function:how estrogen and progesterone regulate the mammary epithelial hierarchy. J Mammary Gland Biol Neoplasia. 2015;20:9–25. doi: 10.1007/s10911-015-9337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen T, Li J, Chen J, Song H, Yang C. Anti-hyperplasia effects of Rosa rugosa polyphenols in rats with hyperplasia of mammary gland. Environ Toxicol Pharmacol. 2015;39:990–6. doi: 10.1016/j.etap.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Diep CH, Daniel AR, Mauro LJ, Knutson TP, Lange CA. Progesterone action in breast, uterine, and ovarian cancers. J Mol Endocrinol. 2015;54:R31–53. doi: 10.1530/JME-14-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrara A. Benign breast disease. Radiol Technol. 2011;82:447M–62M. [PubMed] [Google Scholar]
- 5.Gupta D, Gupta V, Marwah N, Gill M, Gupta S, Gupta G, Jain P, Sen R. Correlation of Hormone Receptor Expression with Histologic Parameters in Benign and Malignant Breast Tumors. Iran J Pathol. 2015;10:23–34. [PMC free article] [PubMed] [Google Scholar]
- 6.Hovey RC, Trott JF. Morphogenesis of mammary gland development. Adv Exp Med Biol. 2004;554:219–28. doi: 10.1007/978-1-4757-4242-8_19. [DOI] [PubMed] [Google Scholar]
- 7.Hu JP, Li SS. Mammary hyperplasia: TCM opinions and treatments. Practical Clinical Journal of Integrated Traditional Chinese and Western Medicine. 2015;15(10):92–93. Chinese. [Google Scholar]
- 8.Macias H, Hinck L. Mammary gland development. Wiley Interdiscip Rev Dev Biol. 2012;2012;1(4):533–557. doi: 10.1002/wdev.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mo DH. The status of breast hyperplasia and its related factors among women of childbearing age in urban and suburban. China Modern Medicine. 2011;18:142–142. Chinese. [Google Scholar]
- 10.Mote PA, Bartow S, Tran N, Clarke CL. Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat. 2002;72:163–172. doi: 10.1023/a:1014820500738. [DOI] [PubMed] [Google Scholar]
- 11.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schillace RV, Skinner AM, Pommier RF, O’Neill S, Muller PJ, Naik AM, Hansen JE, Pommier SJ. Estrogen receptor, progesterone receptor, interleukin-6 and interleukin-8 are variable in breast cancer and benign stem/progenitor cell populations. BMC Cancer. 2014;14:733. doi: 10.1186/1471-2407-14-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shyamala G, Yang X, Silberstein G, Barcellos-Hoff MH, Dale E. Transgenic mice carrying an imbalance in the native ratio of A to B forms of progesterone receptor exhibit developmental abnormalities in mammary glands. Proc Natl Acad Sci U S A. 1998;95:696–701. doi: 10.1073/pnas.95.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silvera SA, Rohan TE. Benign proliferative epithelial disorders of the breast:a review of the epidemiologic evidence. Breast Cancer Res Treat. 2008;110:397–409. doi: 10.1007/s10549-007-9740-3. [DOI] [PubMed] [Google Scholar]
- 15.Ulrich U, Pfeifer T, Lauritzen C. Rapid increase in lumbar spine bone density in osteopenic women by high-dose intramuscular estrogen-progestogen injections. A preliminary report. Horm Metab Res. 1994;26:428–31. doi: 10.1055/s-2007-1001723. [DOI] [PubMed] [Google Scholar]


