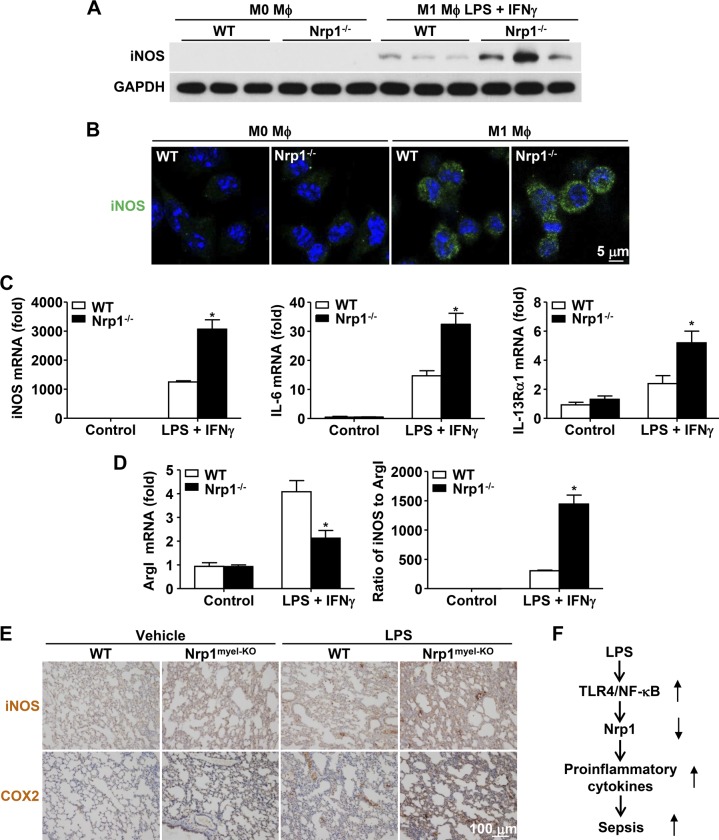
Figure 7.
Nrp1 deficiency induces M1 macrophage polarization. Purified monocytes were incubated for 5 d with M-CSF (50 ng/ml) to differentiate into mature macrophages (M0-phenotype). M0-macrophages were then polarized for an additional 2 d into M1-macrophages by substituting M-CSF for a mixture of LPS (100 ng/ml) and IFN-γ (20 ng/ml). A) Immunoblot analysis of iNOS in WT and Nrp1−/− macrophages. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) serves as loading control. B) Immunofluorescence staining of iNOS (green) in WT and Nrp1−/− BMDMs. Nuclei were stained with DAPI (blue). Scale bar, 5 μm. C) Quantitative RT-PCR analysis of iNOS, IL-6, and IL-13Rα1 mRNA levels. D) ArgI mRNA levels were measured by quantitative RT-PCR, and the ratio of iNOS to ArgI was calculated. E) Immunohistochemical staining of iNOS and COX2 in lung tissue from WT and Nrp1myel-KO mice 20 h after intraperitoneal injection of saline or LPS (20 mg/kg body weight; n = 5 per group). F) Schematic model for LPS-induced Nrp1 loss-exaggerated sepsis. LPS specifically down-regulates Nrp1 mRNA via activation of TLR4–NF-κB pathway in macrophages. Loss or deficiency of Nrp1 augments leads to increased proinflammatory cytokines and causes sepsis. Data are expressed as means ± sem. *P < 0.05 vs. WT.