Abstract
Vitamin B12 deficiency causes megaloblastic anemia and neurologic disorder in humans. Gene defects of transcobalamin (TC) and the transcobalamin receptor (TCblR), needed for cellular uptake of the TC-bound B12, do not confer embryonic lethality. TC deficiency can produce the hematologic and neurologic complications after birth, whereas TCblR/CD320 gene defects appear to produce mild metabolic changes. Alternate maternofetal transport mechanisms appear to provide adequate B12 to the fetus. To understand this mechanism, we evaluated the role of TC, TCblR/CD320, and megalin in maternofetal transport of B12 in a TCblR/CD320-knockout (KO) mouse. Our results showed high expression of TCblR/CD320 in the labyrinth of the placenta, embryonic brain, and spinal column in wild-type (WT) mice. Megalin expression was about the same in both WT and KO mouse visceral yolk sac, brain, and spinal column. Megalin mRNA was down-regulated in the KO embryonic spinal cord (SC) and kidneys. Megalin expression remained unaltered in adult WT and KO mouse brain, SC, and kidneys. Injected dsRed-TC-B12 and TC-57CoB12 accumulated in the visceral yolk sac of KO mice where megalin is expressed and provides an alternate mechanism for the maternofetal transport of Cbl during fetal development.—Arora, K., Sequeira, J. M., Quadros, E. V. Maternofetal transport of vitamin B12: role of TCblR/CD320 and megalin.
Keywords: cobalamin, placenta, fetal development, yolk sac, brain
Vitamin B12 [cobalamin (Cbl)] deficiency in humans causes neuropsychiatric disorder and motor, as well as peripheral neuropathy associated with the demyelination of the SC and peripheral nerves (1, 2). It also leads to defective DNA synthesis and megaloblastic anemia brought on by indirect folate deficiency (3). Although the pathways leading to defective DNA synthesis are defined (3), the metabolic basis for the neuropathology of Cbl deficiency remains unidentified (4). Gene defects in Cbl pathways are not considered to be lethal, because most children with these genetic disorders are carried to term and manifest only the hematologic and neurologic complications of the disorder after birth (5, 6). These infants appear to develop normally in utero but deteriorate rapidly after birth (7). It is presumed that maternal Cbl metabolism compensates for the metabolic abnormality in the fetus (8, 9). This notion is especially true of congenital abnormalities of transcobalamin (TC) involved in the transport and cellular uptake of Cbl (10–13). Untreated, these infants develop megaloblastic anemia, mental retardation, and stunted growth and die in early childhood (5, 6, 11–14). In congenital TC deficiency, maternal TC appears to cross the placental barrier to deliver Cbl to the fetus (9). Gene defects of TCblR/CD320 appear sparse, and only recently, a few cases have been reported (15). The pathologic consequences appear mild, and these children seem to respond well to B12 therapy (15).
To understand the role of TCblR/CD320 in maternofetal transport of Cbl and the compensatory transport process for defects in fetal pathways, we used a TCblR/CD320-knockout (KO) mouse model. The TCblR/CD320 KO is not associated with embryonic lethality, and the homozygous pups are carried to term and appear normal at birth. These mice do not develop systemic Cbl deficiency; they grow normally, and homozygous mice reproduce normally (4). At birth, these mice have normal Cbl levels in all tissues but start losing Cbl in the brain gradually to undetectable levels in a matter of months (4). This results in elevated TC-Cbl in blood without a significant decrease in Cbl in the liver and kidneys. Like the liver, the kidneys also have the ability to accumulate Cbl when a surplus is available in the circulation (16–18). In the absence of TCblR/CD320, an alternate mechanism must operate for tissue and embryonic uptake of Cbl.
Megalin, which is highly expressed during embryonic development, has high affinity for TC-Cbl (19). It is highly expressed in adult kidneys and plays a role in renal filtration, reabsorption, and accumulation of Cbl (16). We hypothesized that megalin is the surrogate receptor for fetal Cbl uptake in the absence of TCblR/CD320. To understand the mechanism by which Cbl is delivered to the developing fetus from maternal blood to fetal CNS, we studied expression of megalin and TCblR/CD320 and maternofetal transport of Cbl in pregnant WT and KO mice. We also analyzed the expression pattern of TCblR/CD320 and megalin in the CNS during fetal development and in the adult mouse. This study was undertaken to gain understanding of the anticipated physiologic function of megalin to deliver Cbl to the developing fetus in the absence of TCblR/CD320.
MATERIALS AND METHODS
Wild-type (WT) C57BL/6J and germline CD320-KO mice crossbred on a C57BL/6J background were used (4). All mice were fed a normal diet (Picolab Rodent Diet 20; LabDiet, St. Louis, MO, USA) containing 50 µg B12 and 3 mg folic acid/kg chow (LabDiet, St. Louis, MO, USA), as recommended by the American Institute of Nutrition (1977), and had free access to food and water. The mice were maintained at 22°C in a 12-h light/dark cycle, and the experimental protocols were approved by the Animal Care and Use Committee of the State University of New York, Downstate Medical Center.
Immunohistochemistry
The expression of TCblR/CD320 and megalin in the WT and TCblR/CD320-KO mice was analyzed by immunohistochemical localization of the proteins at various stages of development and in adult mouse tissues. Paraffin-embedded sections of the embryo in utero (for placenta and yolk sac) from gestational day (GD)15–17, pregnant mice, embryonic day (E)14 and 16 embryos and, adult mouse tissues (brain, SC and, kidneys) were used. Tissues were collected immediately after euthanization and rinsed in cold PBS before they were processed for histology. All tissues were fixed overnight in Carnoy’s solution (ethanol:glacial acetic acid:chloroform; 6:1:3), dehydrated in solutions of increasing ethanol concentrations (70, 90, and 100%), cleared in xylenes (MP Biomedicals, Santa Ana, CA, USA), and embedded in paraplast (Thermo Fisher Scientific, Hampton, NH, USA). Sections (7 µm thick) were prepared with a microtome (Leitz) and mounted on silane‐coated slides. Spinal columns were decalcified and fixed using fixative/decalcifier (VWR, International, Radnor, PA, USA) overnight. The spinal cord was dissected from the bone and embedded in paraffin for sectioning. All the slides with sections were cleared with xylenes, rehydrated in decreasing concentrations of ethanol, and used for immunostaining.
For immunohistochemistry (IHC), the sections were washed with PBS and then incubated with 3% H2O2 in PBS for 5 min. The slides were washed again in PBS (2 times, 5 min each) and incubated with normal horse serum (NHS) for 1 h to reduce nonspecific binding. After removing NHS, primary antibodies were added to the sections, respective to their dilutions. A polyclonal antiserum to mouse TCblR was produced in rabbits by immunizing with a mouse recombinant TCblR-human IgG chimeric protein. Rabbits were injected with 800 μg protein followed by 3 booster injections of 400 μg each. Serum was tested for antibody titer by direct immunoprecipitation of TCblR and by ELISA. The IgG fraction was purified by affinity chromatography on a protein A column and used at 1:500 dilution. For megalin, a polyclonal Ab developed to the cytoplasmic domain polypeptide of megalin (20) was used at 1:300 dilution. The sections were incubated with the primary antiserums for 2 h at room temperature. A dilution of normal rabbit serum similar as to that of the primary antiserum was substituted to serve as a negative control for the tissues examined. After incubation, the preparation was washed with PBS (2 times, 15 min each) and the sections were incubated for 30 min with anti-rabbit IgG coupled to a micropolymer of highly active peroxidase from ImmPress Reagent Kit (Vector Laboratories, Burlingame, CA, USA). The sections were then washed in PBS (two times, 15 min each) followed by reaction with diaminobenzidine to develop the chromogen (Vector Laboratories, Burlingame, CA, USA), in accordance with the manufacturer’s instructions. Each section was then counterstained with methyl green or hematoxylin QS (Vector Laboratories). Slides were permanently mounted with Permount (Thermo Fisher Scientific) after dehydration through ethanol and clearing with xylenes.
DsRed TC-Cbl distribution studies
Recombinant human TC was cloned into pDsRed-N1 plasmid to express a fusion protein with C-terminal DsRed protein (21). The plasmid was transfected into HEK-293 cells and stable clones expressing the fusion protein were selected by culturing cells in medium containing chloramphenicol. The apolipoprotein-dsRed-TC protein was partially purified by chromatography on carboxymethyl cellulose (22), and 0.5 µg protein saturated with Cbl was injected intraperitoneally into GD15–17 pregnant mice. The mice were euthanized after 1 h, and the tissues were collected immediately and processed for detection of the dsRed-TC protein using IHC.
57CoB12 uptake studies
For embryonic uptake of Cbl, GD15–17 mice (WT and KO) were injected with 1 μCi i.p. of 57CoB12. Mice were euthanized at 2 and 24 h after injection, and each embryo in utero was immediately separated. Tissue-specific uptake of 57CoB12 for each tissue (uterus, placenta, yolk sac, and embryo) was measured by recording the radioactivity. Percentage uptake was calculated for each tissue with respect to the total dose injected. The average of the percentage uptake for each tissue was calculated. Student’s t test was used to determine significant differences and P < 0.05 indicated statistical significance.
Adult mice were injected with 1 μCi 57CoB12 and were euthanized 24 h after injection. Different regions of brain, SC, and kidneys were immediately collected, and radioactivity (counts per minute) was measured. The wet weight of each tissue was used to calculate distribution of 57CoB12 as counts per minute per gram tissue.
Quantitative RT-PCR for TCblR/CD320 and megalin transcript
For quantitative PCR (qPCR), tissues were removed, immediately rinsed in cold PBS, and placed in Trizol reagent (Thermo Fisher Scientific). In case of embryonic tissues (brain, spinal column, heart, kidneys), each tissue from 4 embryos was pooled for the analysis. Six pooled samples were used for the study. Different regions of the adult mouse brain were dissected immediately after removal and placed directly in Trizol reagent. Tissues (∼100 mg) were homogenized in 1 ml Trizol reagent (Thermo Fisher Scientific), and RNA was isolated according to the manufacturer’s protocol. Total RNA (5 µg) was used for cDNA synthesis with the RT2 First Strand Kit (Qiagen, Hilden, Germany). qRT-PCR was performed with 0.5 μg total cDNA along with RT2 SYBR Green ROX qPCR Mastermix and gene-specific primers for mouse megalin (forward: 5′-AGGCCACCAGTTCACTTGCT-3′, reverse: 5′-AGGACACGCCCATTCTCTTG-3′), mouse TCblR/CD320 (forward: 5′-GGTCCAAGTCTCCGGCTCTA-3′, reverse: 5′-AGCACATGACTCAATCCTACAGT-3′), and the housekeeping gene mouse β-actin (forward: 5′-GGCTGTATTCCCCTCCATCG-3′, reverse: 5′- CCAGTTGGTAACAATGCCATGT-3′). qRT-PCR was performed in an ABI StepOne instrument (Thermo Fisher Scientific). Amplification cycles included 1 cycle at 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 65°C for 60 s. Ct values obtained were used to calculate relative mRNA expression (2−∆∆Ct). The nonparametric Mann-Whitney U test was used to compare the mean relative mRNA levels from each set, and P < 0.05 was considered to be statistically significant.
RESULTS
TCblR/CD320 and megalin expression in the placenta and yolk sac
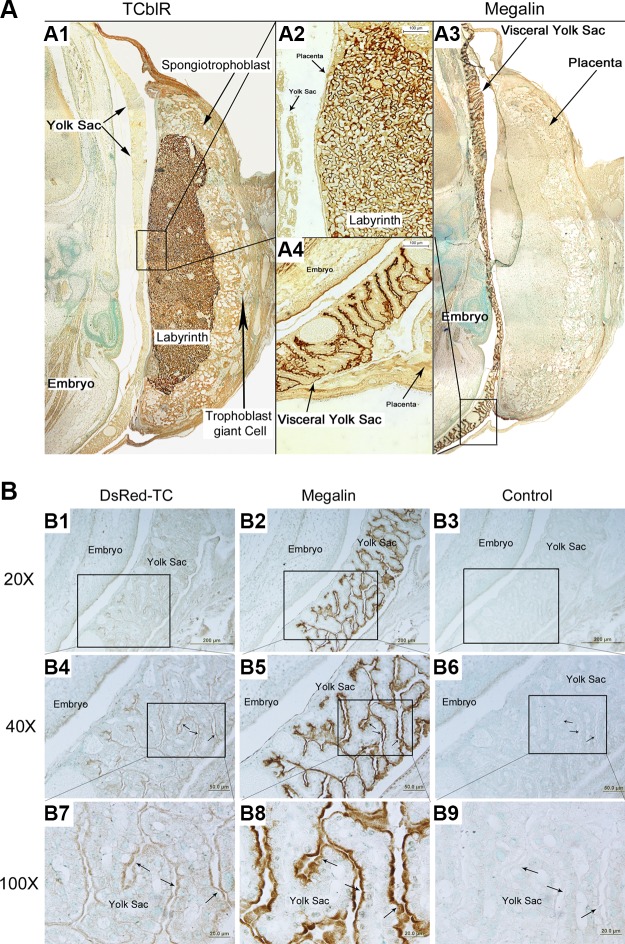
To understand how Cbl is transported from mother to fetus under normal conditions and in the absence of TCblR/CD320, we studied the expression of TCblR/CD320 and megalin in GD15–17 embryos in utero, particularly in placenta and yolk sac. TCblR/CD320 expression was found in the labyrinth of placenta (Fig. 1A1, 2) in trophoblast cells of the WT mice. No TCblR/CD320 expression was found in the homozygous KO mothers and fetuses. High expression of megalin was found in the villous region of the visceral yolk sac (Fig. 1A3, 4) of WT and KO mice. Megalin expression was confined to the apical region of the endodermal epithelium that is in direct contact with the maternal blood. Tissue specific expression of megalin was not altered in the KO mice and appeared to be same as in WT.
Figure 1.
TCblR/CD320 and megalin expression in reproductive tissues of WT pregnant mice. A) TCblR/CD320 is expressed in the trophoblast cells on the fetal side of the placenta in the labyrinth (A1, 2). Megalin is expressed in the visceral yolk sac, specifically toward the apical side of the endodermal epithelium (A3, 4; n = 10). B) In the TCblR/CD320-KO mice (n = 6), colocalization of injected dsRed-TC was found on the apical side of the epithelial layer of yolk sac (B1), where megalin is expressed (B2). Magnification: ×40 (B1, 4); ×100 (B7). Insets: ×40 (B5); ×100 (B8). B3, 6, and 9 were respective negative controls (n = 6).
DsRed-TC-bound Cbl uptake
DsRed-TC-bound Cbl was injected in pregnant TCblR/CD320-KO mice to track its binding to its receptor for uptake. Tissues examined 1 h after injection showed localization of dsRed-TC to the villous region of the visceral yolk sac (Fig. 1B1, 4, 7), precisely where megalin is expressed (Fig. 1B2, 5, 8).
57CoB12 uptake in embryo in utero
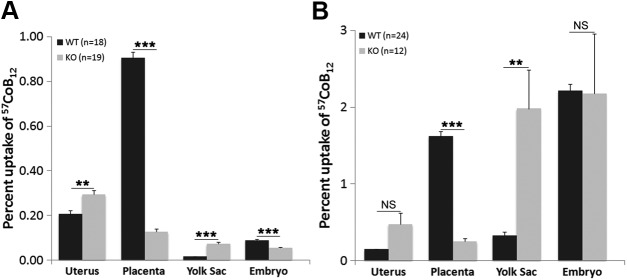
Data were arranged following the order of transport from mother to fetus in utero. In 2 h after, injection, the uterus of TCblR/CD320-KO mice showed an accumulation of injected 57CoB12 similar to that in the WT (Fig. 2A). In the placenta, the WT (expressing TCblR/CD320) showed highest uptake, whereas the TCblR/CD320 KO showed significantly lower 57CoB12 uptake. However, TCblR/CD320 KO showed a relatively high amount of uptake in yolk sac compared to WT, although it was not as high as in WT placenta. Consequently, in 2 h, the TCblR/CD320-KO embryos received lower 57CoB12 compared to WT embryos. However, this was corrected with time. In 24 h after injection, TCblR/CD320-KO placenta still had lower uptake compared to the WT (Fig. 2B). However, yolk sac uptake in the KO increased to levels comparable to that in WT placenta. The TCblR/CD320-KO embryos therefore received amounts of 57CoB12 similar to those of WT in 24 h.
Figure 2.
Distribution of IP injected 57CoB12 in utero, placenta yolk sac, and embryo. Percentage uptake (means ± sem) with respect to the total dose was determined in each organ and the embryo at 2 h (A; WT: n = 18, KO: n = 19) and 24 h (B; WT: n = 24, KO: n = 12) after injection. A) Placenta showed the highest amount of uptake in WT at 2 h. KO lacking TCblR/CD320 in placenta showed a higher percentage uptake in the yolk sac and uterus. KO embryos had a lower uptake compared to WT. B) The uptake in the KO yolk sac increased even further after 24 h (**P < 0.01), and total uptake was similar in WT and KO embryos. **P < 0.01, ***P < 0.001.
TCblR/CD320 and megalin mRNA levels in embryos
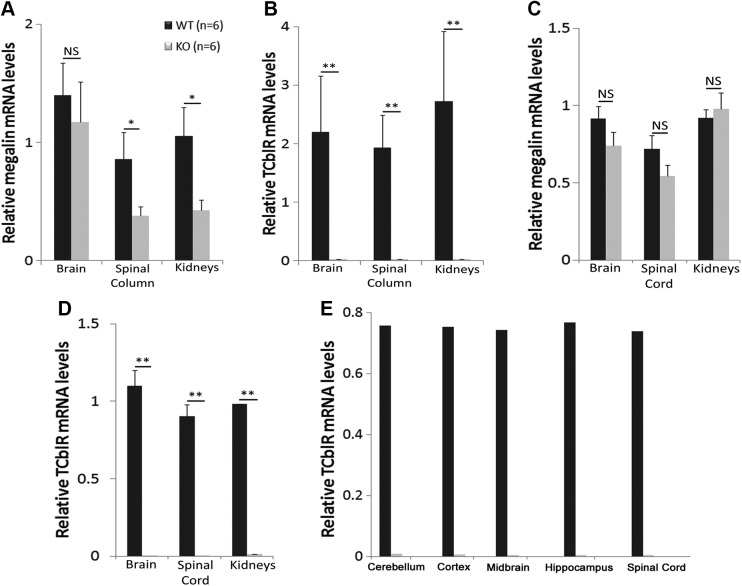
TCblR/CD320 and megalin mRNA expression was studied by qRT-PCR in WT and TCblR/CD320-KO mice. We studied embryonic brain, spinal column, and kidneys of E16 embryos and 7-mo-old adults, to identify any compensatory changes in the expression levels of megalin because of the absence of TCblR/CD320.
In embryos, the mRNA levels of megalin were slightly lower in the brain in TCblR/CD320-KO mice (Fig. 3A), although not significant. Megalin expression was significantly lower in spinal column and kidneys. In contrast, TCblR/CD320 expression was nonexistent in any of the TCblR/CD320-KO mouse tissues, as expected (Fig. 3B).
Figure 3.
Megalin and TCblR/CD320 mRNA levels in WT and KO GD16 fetuses and 6-mo-old adult mice. A) Relative megalin mRNA levels (means ± sem) in brain, spinal column, and kidneys of GD16 KO fetuses were lower compared levels in WT tissues (WT: n = 6, KO: n = 6). B) Relative TCblR/CD320 levels in brain, spinal column, and kidneys of GD16 KO fetuses were not detectable. In adult CNS and kidneys, mean relative megalin mRNA in brain and SC was again lower but not significantly different compared to WT (C; WT: n = 6, KO: n = 6). Relative TCblR/CD320 mRNA levels were not detectable (D). Comparison of mean megalin mRNA (A) with TCblR/CD320 mRNA (B) in WT GD16 fetuses showed similar levels in respective tissues. C, D) Similar results were found in adult tissues. E) TCblR/CD320 mRNA expression was found to be similar in different regions of brain and SC in WT adult mice (WT: n = 2, KO: n = 2). NS, not significant. *P < 0.05, **P < 0.01.
We also compared the megalin mRNA expression with relative TCblR/CD320 mRNA expression in the WT embryonic tissues. Megalin mRNA levels (Fig. 3A) appeared to be expressed at levels similar to TCblR/CD320 mRNA (Fig. 3B), with no significant differences noted.
TCblR/CD320 and megalin mRNA levels in adult mice
Megalin mRNA levels were similar in the brain of WT and KO adult mice (Fig. 3C). The mRNA levels in the SC and kidneys were similar in both groups; SC showed lower but not significantly decreased levels. However, the megalin mRNA levels were increased when compared to the respective embryonic tissues. TCblR/CD320 mRNA was absent in TCblR/CD320-KO adult mouse tissues (Fig. 3D).
Comparison of megalin (Fig. 3C) with TCblR/CD320 (Fig. 3D) in WT adult tissues showed similar expression of both mRNAs.
We also studied TCblR/CD320 mRNA expression in different regions of adult brain to determine its region-specific expression. Our results showed similar amounts of TCblR/CD320 mRNA expression in all regions of the brain (Fig. 3E) in WT mice.
Tissue-specific expression of TCblR/CD320 and megalin during embryonic development
TCblR/CD320 and megalin expression was studied with IHC. We studied E14 and 16 embryos and 7-mo-old adults. The results provided information about tissue-specific expression of TCblR/CD320 and megalin in WT and TCblR/CD320-KO mice and was used to determine how Cbl is further transported to the CNS of embryos.
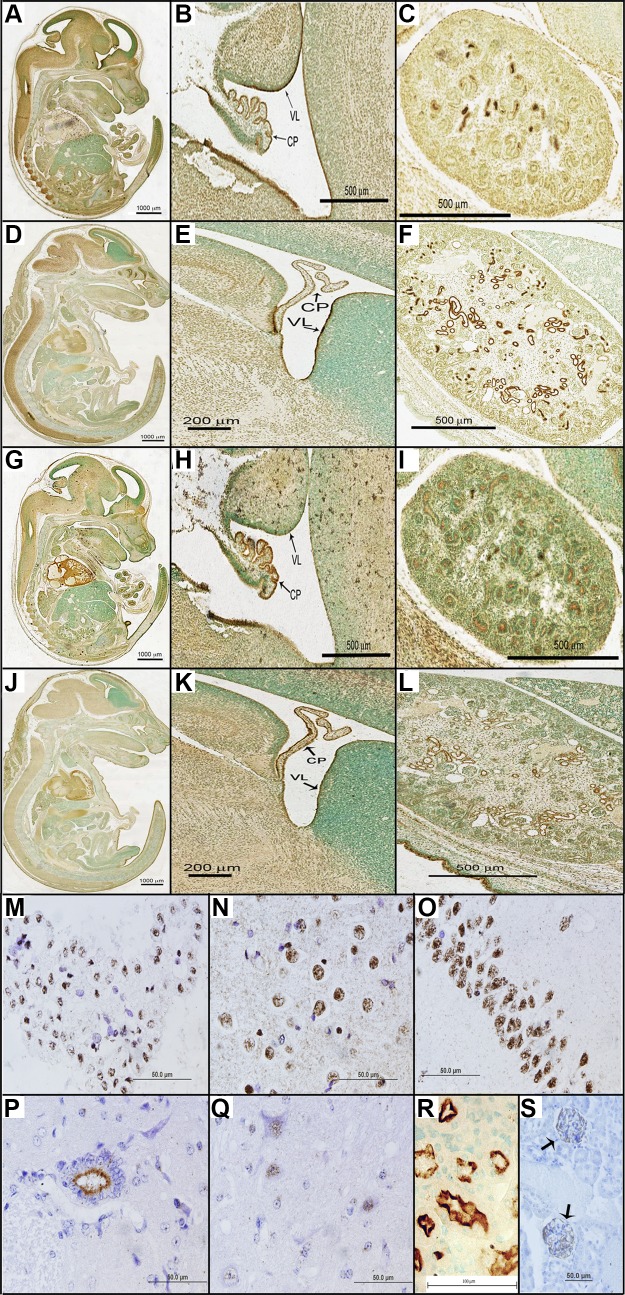
In E14 embryos, megalin expression was found in the whole brain, particularly in the ventricular lining (VL) and choroid plexus (CP) and in the spinal column (Fig. 4A, B). In VL, megalin was expressed in ependymal cells in the apical and basal surfaces. In CP, megalin expression was found in the whole ependymal cell surface with higher expression in the apical region. In kidneys, the expression was observed in the apical surface of the renal tubules throughout the tissue, where TC-Cbl can be reabsorbed (Fig. 4C). In addition, megalin expression was observed in the medial nasal process (MNP) and lateral nasal process (LNP) (Fig. 4A).
Figure 4.
Megalin and TCblR/CD320 expression in E14 (n = 3) and E16 (n = 3) fetal and adult CNS and kidneys. Megalin expression in the embryonic brain was found in the apical and basal regions of ependymal cells of CP and VL (A, B, D, E), in the spinal column (A, D), and in kidneys (C, F). TCblR/CD320 expression in embryos was also found in the apical and basal regions of ependymal cells of CP and VL in the brain (G, H, J, K). TCblR/CD320 expression was also found in kidneys (I, L). In adult mouse brain (WT: n = 8, KO: n = 8), megalin expression was found in the nucleus of cells in choroid plexus (M), cortex (N), and hippocampus (O, CA1 region). In adult spinal cord, megalin expression was found in the ependymal cells on the apical surface toward the central canal (P) and in the nuclear region of neurons (Q). R) In adult kidneys, megalin was expressed in renal tubules. S) TCblR/CD320 expression, in adult kidneys, was found in the corpuscles of the renal cortex.
Megalin in E16 embryos was still expressed in the same regions of the brain as in E14 (Fig. 4D, E). The expression in spinal column extended from cervical to lumbar region (Fig. 4D). Besides the expression in renal tubules of kidneys (Fig. 4F), MNP, and LNP, increased megalin expression was localized to the crypt, enterocytes, and villi of the small intestines (Fig. 4D).
TCblR/CD320 in E14 and E16 embryos appeared to be coexpressed in the same regions as megalin. In E14 WT embryos, TCblR/CD320 expression was found in the VL and CP of brain (Fig. 4G–K), spinal column (Fig. 4G, J), renal tubules in all regions of kidneys (Fig. 4I, L), MNP, and LNP (Fig. 4G, J), as in the case of megalin. In addition, TCblR/CD320 expression was found in the atrial and ventricular myocytes of the heart. Tissue specific expression remained the same in the E16 embryos.
Tissue-specific expression of TCblR/CD320 and megalin in adult mice
In the adult mice, megalin expression was found in the brain, SC, and kidneys. Megalin expression was found in the nucleus of cells in the choroid plexus (Fig. 4M), cortex (Fig. 4N), and the CA1 region of the of the hippocampus (Fig. 4O). Similar expression was observed in the CA2, CA3, and, dentate gyrus regions of the hippocampus (data not shown). In the SC, megalin expression was found in the ependymal cells on the surface toward the central canal (Fig. 4P) and in the nuclear region of neurons (Fig. 4Q). In adult kidneys, megalin was expressed in the distal and proximal tubules; however, the expression was restricted to the cortex region (Fig. 4R). Similarly, TCblR/CD320 expression in adult kidneys was confined to the renal corpuscles in the cortex (Fig. 4S).
Distribution of 57CoB12 in adult CNS and kidneys
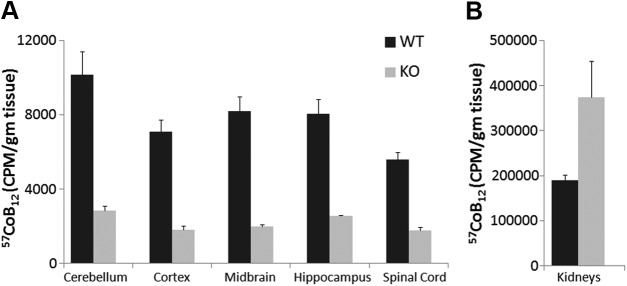
Uptake of 57CoB12 was studied to determine Cbl distribution in different regions of the brain. In TCblR/CD320-KO adult mice, the uptake was very low in all regions of the brain as well as SC, compared to WT (Fig. 5A). In WT mice, 57CoB12 uptake per gram of tissue was similar in all regions of the brain (on average, ∼20–24% of total uptake in the brain) with cerebellum showing slightly higher levels (∼30%). On the other hand, the uptake of 57CoB12 was very high in TCblR/CD320 KO kidneys, as compared to WT (Fig. 5B).
Figure 5.
Distribution (means ± sem) of 57CoB12 in different regions of brain, SC, and kidneys of adult WT (n = 3) and KO (n = 3) mice. A) Injected 57CoB12 was almost equally distributed to all regions of brain and spinal cord (20–30% of total dose accumulated in brain tissue). The radioactivity observed in the KO brain was low and could be related to residual blood in the tissue. B) The KO kidneys showed higher uptake than in WT.
DISCUSSION
Multiple causes contribute to Cbl deficiency because of its complex mechanism of absorption, transport, and utilization (23). TCblR/CD320 is a plasma membrane receptor for TC-Cbl that is required by cells for the uptake of Cbl (24, 25). Although TCblR/CD320 deficiency can cause neurologic disorder, it is not embryonically lethal (4, 26). To understand how TCblR/CD320 is involved in the maternofetal transport of Cbl, we evaluated the process in WT and TCblR/CD320-KO mice.
In a typical case, the maternofetal transport mechanism involves passage of maternal blood from large central arterial sinuses, to the spongiotrophoblasts, and then to the labyrinth. In the labyrinth, fetal trophoblastic villi come in contact with maternal blood for nutrient exchange (27). Cbl (bound to TC), in this region, is transported through receptor-mediated endocytosis (28). Further, fetal vasculature is directly connected to the umbilical cord which transports nutrients to the developing fetus (27). Expression of TCblR/CD320 in trophoblast cells of the labyrinth suggests that TCblR/CD320 is essential for uptake of Cbl from maternal blood and its transfer to the fetus.
Prior studies have shown that the transport of Cbl from mother to fetus involves 3 steps: 1) transport of Cbl from maternal blood to the maternal side of the placenta, 2) transfer from the maternal to the fetal side of the placenta and 3) delivering Cbl from the fetal side of the placenta to the fetal tissues. In this process, steps 2 and 3 are known to be rate limiting (29). Our results indicate that, in typical conditions, TC-Cbl binds to TCblR/CD320 with high affinity (25), and the process facilitates its transport to the embryos, making step 2 the rate-limiting step with transient accumulation of TC-Cbl at the junction between maternal and fetal separation.
Like the placenta, the visceral yolk sac is vascularized by peripheral vitelline circulation of the maternal blood and is known to be involved in receptor-mediated endocytosis (30). The visceral yolk sac is further connected to the umbilical cord (30). The yolk sac, specifically the visceral yolk sac, is known to provide a maternofetal transport system in cases of dysfunctional placenta (31–34). Megalin, coincidently expressed in this region of the yolk sac (35), is a multiligand receptor that binds TC-Cbl and serves an important function in its renal tubular reabsorption (19, 36). The high expression of megalin in the visceral yolk sac, localization of TC, and 57CoB12 accumulation in the same region in TCblR/CD320-KO mice support the role of megalin in fetal delivery of Cbl. Altogether; our data suggest that in the absence of TCblR/CD320, the functional role of placenta in transport of Cbl is no longer present. In this case, the yolk sac provides an alternate mechanism of Cbl transport through megalin.
Megalin is highly expressed in embryonic development, and the knockout is lethal (37). It is essential for development of the nervous system (38, 39). Megalin mRNA expression was down-regulated in the spinal column and kidneys of TCblR/CD320-KO fetuses, but not significantly in the adults. Studies have shown down-regulation of megalin via the LPS-TNF-α-ERK1/2 pathway with higher expression of TNF-α in these cases (40). TNF-α, a proinflammatory cytokine, is a major component of the neuroinflammatory response in CNS diseases associated with Cbl deficiency (41, 42). Elevation of this cytokine could occur because of homocysteine, a known neurotoxin (43), which is elevated in Cbl deficiency (4, 44). TCblR/CD320-KO adult mice show elevated homocysteine in the brain and SC (4). However, expression of megalin in maternofetal tissues suggests a role for megalin in Cbl transport during fetal development in TCblR/CD320-KO mice. After development, megalin expression (of cytoplasmic polypeptide) gets confined to the nucleus in the CNS, possibly because of its role in transcriptional regulation (45). Therefore, Cbl transport in KO adult mouse CNS is diminished, and, as a consequence, Cbl deficiency ensues (4). Our results show relatively low uptake of Cbl in the brain, similar to the level of this vitamin in the CSF, amounting to one-tenth of that in serum (46, 47). Incidentally, soluble TCblR/CD320 identified in CSF is similar in concentration to the TC-Cbl (48), but there is considerable apo-TC in the CSF (46, 47). Even though the precise mechanism of Cbl uptake across the blood–brain barrier has not been elucidated, uptake of TC-Cbl by the ependymal cells lining the ventricles is a plausible mechanism. However, vesicular transport of TCblR-TC-Cbl via the CSF, as well as the microvasculature of the brain parenchyma cannot be excluded. Megalin expression is not decreased in the KO adult kidneys, where it is still expressed on the cell surface of renal tubules. The elevated TC-Cbl in blood (4) is a strong indication of the lack of TC-Cbl uptake in the CNS and perhaps explains the high Cbl accumulation in the kidneys caused by renal reabsorption of TC-Cbl by megalin. This study provides evidence supporting the participation of megalin in Cbl transport to developing fetuses in the TCblR/CD320-KO mice. The surrogate role of megalin in embryonic uptake of Cbl also explains why the gene KO is not lethal and why fetal development is not affected in human gene disorders of TC and TCblR/CD320.
AUTHOR CONTRIBUTIONS
K. Arora, J. M. Sequeira, and E. V. Quadros designed the study; K. Arora and J. M. Sequeira performed the experiments; and all authors contributed to the interpretation of the data and wrote the manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Scott Argraves (Medical University of South Carolina, Charleston, SC, USA) for the generous gift of antibody to megalin. This work was supported, in part, by U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant DK064732 (to E.V.Q.). The recombinant mouse TCblR was a gift from Dr. Jeffrey Browning (Biogen-Idec, Cambridge MA, USA). The authors declare no conflicts of interest.
Glossary
- Cbl
cobalamin (vitamin B12)
- CP
choroid plexus
- CPM
counts per minute
- CSF
cerebrospinal fluid
- E
embryonic day
- GD
gestational day
- IHC
immunohistochemistry
- KO
knockout
- LNP
lateral nasal process
- MNP
medial nasal process
- qPCR
quantitative PCR
- SC
spinal cord
- NHS
normal horse serum
- TC
transcobalamin
- TCblR
transcobalamin receptor
- VL
ventricular lining
- WT
wild type
REFERENCES
- 1.Lachner C., Steinle N. I., Regenold W. T. (2012) The neuropsychiatry of vitamin B12 deficiency in elderly patients. J. Neuropsychiatry Clin. Neurosci. 24, 5–15 [DOI] [PubMed] [Google Scholar]
- 2.Shipton M. J., Thachil J. (2015) Vitamin B12 deficiency: a 21st century perspective. Clin. Med. (Lond.) 15, 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chanarin I., Deacon R., Lumb M., Muir M., Perry J. (1985) Cobalamin-folate interrelations: a critical review. Blood 66, 479–489 [PubMed] [Google Scholar]
- 4.Lai S. C., Nakayama Y., Sequeira J. M., Wlodarczyk B. J., Cabrera R. M., Finnell R. H., Bottiglieri T., Quadros E. V. (2013) The transcobalamin receptor knockout mouse: a model for vitamin B12 deficiency in the central nervous system. FASEB J. 27, 2468–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenblatt D. S., Cooper B. A., Schmutz S. M., Zaleski W. A., Casey R. E. (1985) Prenatal vitamin B12 therapy of a fetus with methylcobalamin deficiency (cobalamin E disease). Lancet 325, 1127–1129 [DOI] [PubMed] [Google Scholar]
- 6.Shevell M. I.,, Rosenblatt D. S. (1992) The neurology of cobalamin. Can. J. Neurol. Sci. 19, 472–486 [PubMed] [Google Scholar]
- 7.Rasmussen S. A., Fernhoff P. M., Scanlon K. S. (2001) Vitamin B12 deficiency in children and adolescents. J. Pediatr. 138, 10–17 [DOI] [PubMed] [Google Scholar]
- 8.Obeid R., Morkbak A. L., Munz W., Nexo E., Herrmann W. (2006) The cobalamin-binding proteins transcobalamin and haptocorrin in maternal and cord blood sera at birth. Clin. Chem. 52, 263–269 [DOI] [PubMed] [Google Scholar]
- 9.Quadros E. V. (2010) Advances in the understanding of cobalamin assimilation and metabolism. Br. J. Haematol. 148, 195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasad C., Rosenblatt D. S., Corley K., Cairney A. E., Rupar C. A. (2008) Transcobalamin (TC) deficiency: potential cause of bone marrow failure in childhood. J. Inherit. Metab. Dis. 31(Suppl 2), S287–S292 [DOI] [PubMed] [Google Scholar]
- 11.Ratschmann R., Minkov M., Kis A., Hung C., Rupar T., Mühl A., Fowler B., Nexo E., Bodamer O. A. (2009) Transcobalamin II deficiency at birth. Mol. Genet. Metab. 98, 285–288 [DOI] [PubMed] [Google Scholar]
- 12.Ünal Ş., Rupar T., Yetgin S., Yaralı N., Dursun A., Gürsel T., Çetin M. (2015) Transcobalamin II deficiency in four cases with novel mutations. Turk. J. Haematol. 32, 317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ünal S., Tezol O., Oztas Y. (2014) A novel mutation of the transcobalamin II gene in an infant presenting with hemophagocytic lymphohistiocytosis. Int. J. Hematol. 99, 659–662 [DOI] [PubMed] [Google Scholar]
- 14.Kömhoff M., Roofthooft M. T., Westra D., Teertstra T. K., Losito A., van de Kar N. C., Berger R. M. (2013) Combined pulmonary hypertension and renal thrombotic microangiopathy in cobalamin C deficiency. Pediatrics 132, e540–e544 [DOI] [PubMed] [Google Scholar]
- 15.Quadros E. V., Lai S. C., Nakayama Y., Sequeira J. M., Hannibal L., Wang S., Jacobsen D. W., Fedosov S., Wright E., Gallagher R. C., Anastasio N., Watkins D., Rosenblatt D. S. (2010) Positive newborn screen for methylmalonic aciduria identifies the first mutation in TCblR/CD320, the gene for cellular uptake of transcobalamin-bound vitamin B(12). Hum. Mutat. 31, 924–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birn H., Willnow T. E., Nielsen R., Norden A. G., Bönsch C., Moestrup S. K., Nexø E., Christensen E. I. (2002) Megalin is essential for renal proximal tubule reabsorption and accumulation of transcobalamin-B(12). Am. J. Physiol. Renal Physiol. 282, F408–F416 [DOI] [PubMed] [Google Scholar]
- 17.Harte R. A., Chow B. F., Barrows L. (1953) Storage and elimination of vitamin B12 in the rat. J. Nutr. 49, 669–678 [DOI] [PubMed] [Google Scholar]
- 18.Scott J. S., Treston A. M., Bowman E. P., Owens J. A., Cooksley W. G. (1984) The regulatory roles of liver and kidney in cobalamin (vitamin B12) metabolism in the rat: the uptake and intracellular binding of cobalamin and the activity of the cobalamin-dependent enzymes in response to varying cobalamin supply. Clin. Sci. 67, 299–306 [DOI] [PubMed] [Google Scholar]
- 19.Moestrup S. K., Birn H., Fischer P. B., Petersen C. M., Verroust P. J., Sim R. B., Christensen E. I., Nexø E. (1996) Megalin-mediated endocytosis of transcobalamin-vitamin-B12 complexes suggests a role of the receptor in vitamin-B12 homeostasis. Proc. Natl. Acad. Sci. USA 93, 8612–8617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drake C. J., Fleming P. A., Larue A. C., Barth J. L., Chintalapudi M. R., Argraves W. S. (2004) Differential distribution of cubilin and megalin expression in the mouse embryo. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 277A, 163–170 [DOI] [PubMed] [Google Scholar]
- 21.Jiang W., Nakayama Y., Sequeira J. M., Quadros E. V. (2011) Characterizing monoclonal antibodies to antigenic domains of TCblR/CD320, the receptor for cellular uptake of transcobalamin-bound cobalamin. Drug Deliv. 18, 74–78 [DOI] [PubMed] [Google Scholar]
- 22.Quadros E. V., Rothenberg S. P., Pan Y. C., Stein S. (1986) Purification and molecular characterization of human transcobalamin II. J. Biol. Chem. 261, 15455–15460 [PubMed] [Google Scholar]
- 23.Dali-Youcef N.,, Andres E. (2009) An update on cobalamin deficiency in adults. QJM 102, 17–28 [DOI] [PubMed] [Google Scholar]
- 24.Lai S. C., Nakayama Y., Sequeira J. M., Quadros E. V. (2011) Down-regulation of transcobalamin receptor TCblR/CD320 by siRNA inhibits cobalamin uptake and proliferation of cells in culture. Exp. Cell Res. 317, 1603–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quadros E. V., Nakayama Y., Sequeira J. M. (2009) The protein and the gene encoding the receptor for the cellular uptake of transcobalamin-bound cobalamin. Blood 113, 186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCaddon A. (2013) Vitamin B12 in neurology and ageing; clinical and genetic aspects. Biochimie 95, 1066–1076 [DOI] [PubMed] [Google Scholar]
- 27.Rossant J., Cross J. C. (2001) Placental development: lessons from mouse mutants. Nat. Rev. Genet. 2, 538–548 [DOI] [PubMed] [Google Scholar]
- 28.Fernandes-Costa F., Metz J. (1979) Transplacental transport in the rabbit of vitamin B12 bound to human transcobalamin I, II and III. Br. J. Haematol. 43, 625–630 [DOI] [PubMed] [Google Scholar]
- 29.Graber S. E., Scheffel U., Hodkinson B., McIntyre P. A. (1971) Placental transport of vitamin B12 in the pregnant rat. J. Clin. Invest. 50, 1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jollie W. P. (1990) Development, morphology, and function of the yolk-sac placenta of laboratory rodents. Teratology 41, 361–381 [DOI] [PubMed] [Google Scholar]
- 31.Lerman S., Koszalka T. R., Jensen M., Andrew C. L., Beckman D. A., Brent R. L. (1986) In vitro studies on the effect of yolk sac antisera on functions of the visceral yolk sac, I: pinocytosis and transport of small molecules. Teratology 34, 335–341 [DOI] [PubMed] [Google Scholar]
- 32.Lloyd J. B., Beckman D. A., Brent R. L. (1998) Nutritional role of the visceral yolk sac in organogenesis-stage rat embryos. Reprod. Toxicol. 12, 193–195 [DOI] [PubMed] [Google Scholar]
- 33.Mossman H. W. (1987) Vertebrate Fetal Membranes: Comparative Ontogeny and Morphology, Evolution, Phylogenetic Significance, Basic Functions, Research Opportunities. Rutgers University Press, New Brunswick, NJ, USA: [Google Scholar]
- 34.Waddell W. J., Marlowe C. (1981) Transfer of drugs across the placenta. Pharmacol. Ther. 14, 375–390 [DOI] [PubMed] [Google Scholar]
- 35.Zheng G., Bachinsky D. R., Stamenkovic I., Strickland D. K., Brown D., Andres G., McCluskey R. T. (1994) Organ distribution in rats of two members of the low-density lipoprotein receptor gene family, gp330 and LRP/alpha 2MR, and the receptor-associated protein (RAP). J. Histochem. Cytochem. 42, 531-542 [DOI] [PubMed] [Google Scholar]
- 36.Christensen E. I., Verroust P. J. (2002) Megalin and cubilin, role in proximal tubule function and during development. Pediatr. Nephrol. 17, 993–999 [DOI] [PubMed] [Google Scholar]
- 37.Willnow T. E., Hilpert J., Armstrong S. A., Rohlmann A., Hammer R. E., Burns D. K., Herz J. (1996) Defective forebrain development in mice lacking gp330/megalin. Proc. Natl. Acad. Sci. USA 93, 8460–8464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarthy R. A., Argraves W. S. (2003) Megalin and the neurodevelopmental biology of sonic hedgehog and retinol. J. Cell Sci. 116, 955–960 [DOI] [PubMed] [Google Scholar]
- 39.Wicher G., Aldskogius H. (2008) Megalin deficiency induces critical changes in mouse spinal cord development. Neuroreport 19, 559–563 [DOI] [PubMed] [Google Scholar]
- 40.Takeyama A., Sato H., Soma-Nagae T., Kabasawa H., Suzuki A., Yamamoto-Kabasawa K., Hosojima M., Kaneko R., Higuchi F., Kaseda R., Ogasawara S., Narita I., Saito A. (2011) Megalin is downregulated via LPS-TNF-α-ERK1/2 signaling pathway in proximal tubule cells. Biochem. Biophys. Res. Commun. 407, 108–112 [DOI] [PubMed] [Google Scholar]
- 41.Olmos G., Lladó J. (2014) Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm. 2014, 861231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scalabrino G. (2009) The multi-faceted basis of vitamin B12 (cobalamin) neurotrophism in adult central nervous system: lessons learned from its deficiency. Prog. Neurobiol. 88, 203–220 [DOI] [PubMed] [Google Scholar]
- 43.Lipton S. A., Kim W. K., Choi Y. B., Kumar S., D’Emilia D. M., Rayudu P. V., Arnelle D. R., Stamler J. S. (1997) Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. USA 94, 5923–5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stabler S. P., Allen R. H., Savage D. G., Lindenbaum J. (1990) Clinical spectrum and diagnosis of cobalamin deficiency. Blood 76, 871–881 [PubMed] [Google Scholar]
- 45.Wicher G., Larsson M., Rask L., Aldskogius H. (2005) Low-density lipoprotein receptor-related protein (LRP)-2/megalin is transiently expressed in a subpopulation of neural progenitors in the embryonic mouse spinal cord. J. Comp. Neurol. 492, 123–131 [DOI] [PubMed] [Google Scholar]
- 46.Hansen M., Brynskov J., Christensen P. A., Krintel J. J., Gimsing P. (1985) Cobalamin binding proteins (haptocorrin and transcobalamin) in human cerebrospinal fluid. Scand. J. Haematol. 34, 209–212 [DOI] [PubMed] [Google Scholar]
- 47.Lazar G. S., Carmel R. (1981) Cobalamin binding and uptake in vitro in the human central nervous system. J. Lab. Clin. Med. 97, 123–133 [PubMed] [Google Scholar]
- 48.Abuyaman O., Nexo E. (2015) The soluble transcobalamin receptor (sCD320) is present in cerebrospinal fluid and correlates to dementia-related biomarkers tau proteins and amyloid-beta. Scand. J. Clin. Lab. Invest. 75, 514–518 [DOI] [PubMed] [Google Scholar]