Fig. 1.
Regulation of IP3Rs by cAMP signalling junctions.
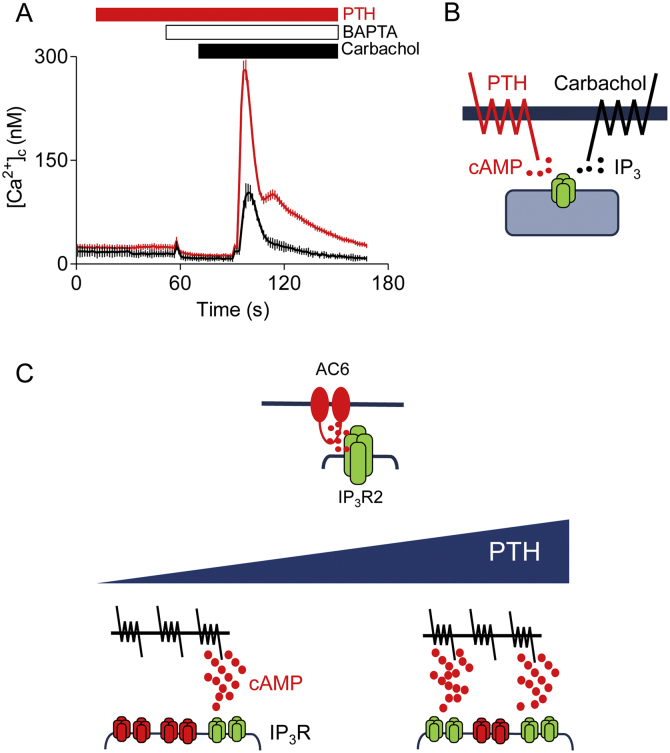
(A) Populations of fluo4-loaded HEK-293 cells stably expressing type 1 receptors for PTH were stimulated with PTH (100 nM, red line) and carbachol (20 μM, both lines) as indicated after addition of BAPTA to chelate extracellular Ca2+. Results show that PTH does not alone evoke an increase in [Ca2+]c, but it potentiates the Ca2+ signal evoked by carbachol. Similar results are shown in Refs. [36], [39]. (B) In HEK-293 cells, PTH through heterologously expressed type 1 PTH receptors stimulates adenylyl cyclase and so formation of cAMP. Carbachol stimulates endogenous M3 muscarinic receptors, which activate PLC and thereby formation of IP3 and release of Ca2+ from the ER through IP3Rs. The potentiation of carbachol-evoked Ca2+ signals by PTH is entirely mediated by cAMP, which enhances IP3-evoked Ca2+ release from IP3Rs. (C) IP3R2 and AC6 are selectively associated at cAMP signalling junctions. Within these junctions, cAMP is delivered from AC to IP3Rs at concentrations far greater than needed to maximally sensitize the associated IP3Rs. This allows each junction to function as a digital ‘on-off’ switch, it ensures that cAMP-mediated signalling operates with a considerable safety margin, and it allows rapid activation of the associated IP3Rs (as cAMP is locally delivered at high concentration) and rapid de-activation (as diffusion of cAMP form the junction reduces its concentration to below that needed for sensitizing IP3Rs). Since each signalling junction operates as an ‘on-off’ switch, the concentration-dependent effects of PTH are proposed to be due to recruitment of junctions, rather than to graded activity within individual junctions.