Summary
Background
The immune response in patients with melanoma is an important focus of research due to the tumor’s resistance and immunotherapy possibilities. IL-27 is one of the cytokines with antitumor properties. The role of IL-27 in the pathogenesis of melanoma is still unclear. The aim of this study was to examine the association between serum IL-27 levels and the clinical parameters of melanoma patients.
Methods
The IL-27 concentration was determined by com mercial ELISA in serum samples from melanoma patients (n=72) and healthy control subjects (n=44). Patients were classified according to AJCC clinical stage, TNM stage, the length of progression-free interval (PFI) and the extent of the disease (localized or widespread).
Results
Average IL-27 values were increased in patients with early stages of melanoma compared to patients with terminal stages and control values. The highest IL-27 concentration was found in stage IIa. Patients in stages III and IV had significantly lower values of IL-27 compared to control. Patients with localized melanoma and shorter PFI had insignificantly increased IL-27 levels compared to patients with widespread disease and longer PFI. Patients with metastatic disease and stage TNM4 had significantly lower average IL-27 values compared to control. Patients with high production of IL-27 (>1000 pg/mL) were most numerous in IIa AJCC stage, with initial tumor size TNM2 and in the group of patients with localized disease.
Conclusions
High levels of IL-27 in patients with melanoma are associated with the initial stages and lo calized disease.
Keywords: antitumor response, interleukin 27, melanoma
Kratak sadržaj
Uvod
Povećana incidenca melanoma danas predstavlja jedan od vodećih medicinskih problema. Imunski odgovor u bolesnika sa melanomom predstavlja važn fokus istraživanja, zbog rezistentnosti ovog tumora na postojeće vidove terapije i mogućnosti imunoterapije. IL-27 je jedan od citokina sa antitumorskim svojstvima. Uloga IL-27 u patogenezi melanoma još uvek nije dovoljno rasvetljena. Cilj ove studije bio je da se ispita povezanost vrednosti IL-27 u serumu sa kliničkim parametrima bolesnika sa melanomom.
Metode
Koncentracija IL-27 određena je komercijalnim ELISA testom u uzorcima seruma bolesnika sa melanomom (n=72) i zdravih kontrolnih osoba (n=44). Bolesnici su klasifikovani prema AJCC kliničkom stadijumu, TNM stadijumu, dužini intervala bez progresije bolesti (PFI) i na osnovu proširenosti bolesti (lokalizovana ili raširena).
Rezultati
Prosečne vrednosti IL-27 bile su povećane u bolesnika sa početnim stadijumima melanoma u odnosu na bolesnike sa terminalnim stadijumima i kontrolne vrednosti. Koncentracija IL-27 je bila značajno najveća u stadijumu IIa. Bolesnici u III i IV stadijumu imali su značajno niže vrednosti IL-27 u odnosu na kontrolne. Bolesnici sa lokalizovanim melanomom i kraćim intervalom bez progresije bolesti imali su neznačajno povećane vrednosti u odnosu na bolesnike sa metastazama i dužim intervalom bez progresije bolesti. Bolesnici sa metastazama i TNM4 stadijumom imali su značajno niže prosečne vrednosti IL-27 u odnosu na kontrolne. Pacijenti sa visokom produkcijom IL-27 (>1000 pg/mL) bili su najbrojniji u IIa AJCC stadijumu, sa početnom veličinom tumora TNM2 i u grupi bolesnika sa lokalizovanom bolešću.
Zaključak
Visoke vrednosti IL-27 kod bolesnika sa melanomom povezane su sa početnim stadijumima i lokalizovanom boleušć.
Introduction
Melanoma is a disease that has affected mankind for a long time. It has been identified in Inca mummies in Peru more than 2000 years old. The term melanoma is derived from the Greek words »meals« meaning black and »oma« meaning tumor. According to the World Health Organization (WHO) classification, there are four common types of melanomas: superficial spreading, nodular, lentigo maligna and acral lentiginous (1). Although melanoma accounts for only 4% of all skin cancers, it causes the greatest number of skin cancer related deaths worldwide. Melanoma usually affects Caucasians in the 4th decade of life, most commonly located on the back of males and the legs of females. Risk factors for the development of melanoma are pale skin, blond or red hair, numerous freckles and tendency to burn and tan poorly, presence of more than 50 acquired naevi, more than five displastic naevi, chemical exposures, immunosuppression, genetic factors, scars etc. Intermittent sun exposure seems to be an important factor (2). Episodic exposure of fair-skinned individuals to intense sunlight is thought to be responsible for the steadily increasing melanoma incidence worldwide over recent decades.
Immune response to melanoma cells is well documented, both in experimental models and in patients (3). Spontaneous or therapy induced melanoma regression confirms the importance of immune control over malignant melanocytes (4). This complex interaction between malignant and immune cells is regulated by numerous membrane molecules and soluble mediators. One of them is IL-27. IL-27 is a heterodimeric cytokine of the IL-6/IL-12 family and consists of an EBV transformed gene 3 (IL-12p40-related protein) and p28 (IL-12p35-related protein) (5). APCs are considered as the main source of IL-27 (6). The expression of IL-27 has also been detected in monocytes, endothelial cells, dendritic cells and trophoblast cells (5, 7). The IL-27 receptor is composed of IL- 27Rα (also known as WSX-1 and TCCR) and gp130 (8). WSX-1 is expressed primarily in lymphoid tissues and its expression is highest in naive T cells and NK cells, monocytes, mast cells and neutrophils (8, 9). IL- 27 plays important roles in the early regulation of Th1 differentiation but also as an inhibitor of the immune response (10). Moreover, IL-27 stimulates rapid clonal expansion of naive CD4+T cells and synergizes with IL-12 to trigger IFN-γ production in naive CD4+T cells and NK cells (5). Activation of IL-27R induces phosphorylation of STAT 1,2,3,4 and 5 (11) and augments T-bet expression through STAT1 activation (12). T-bet stimulates Th1 response by transactivating the IFN-γ gene. IL-27 can directly activate CD4+ and CD8+T cells, and induce their proliferation and differentiation through activation of STAT3 and STAT1 (12,13) which is independent of IFN-γ (14).
It has been reported that IL-27 has the ability to induce tumor specific antitumor and protective immunity using colon carcinoma (14, 15) and TBJ neuroblastoma (16). Motomu Shimizu et al. showed in their study (17) that IL-27 possesses antiangiogenic activity. Moreover, they showed the ability of IL-27 to inhibit tumor growth and metastasis of murine melanoma, as well as induction of antiangiogenic chemokines. IL-27 may be a candidate as an antitumor agent for cancer immunotherapy thanks to its multiple mechanisms, induction of CD8+T cells and NK cells, antiangiogenic and antiproliferative activity.
In this study, we have investigated the association of IL-27 levels with clinical parameters in melanoma patients.
Materials and Methods
Patients and healthy controls
Melanoma patients were recruited from the Clinic of Dermatovenerology and Melanoma Center of the Military Medical Academy, Medical Faculty, University of Defense, Ministry of Defense, Belgrade, Serbia. Healthy controls were recruited during periodical systematic examinations of apparently healthy persons, with no prior history of cancer. All patients and healthy controls were consented and this study was approved by the local Research Ethics Committee, Military Medical Academy (11-03/2014). The study included 72 melanoma patients (35 men and 37 women), and 44 healthy controls (21 men and 23 women). Melanoma patients were classified according to the 7th edition of the American Joint Committee on Cancer (AJCC) classification for melanoma. Characteristics of melanoma patients and controls are shown in Table I.
Table I.
Characteristics of investigated patients and controls
| Subjects characteristics | ||
|---|---|---|
| Patients (n) | Male | 35 |
| Female | 37 | |
| Healthy controls (n) | Male | 21 |
| Female | 23 | |
| Average age (years) | Patients | 54.71±16.61 |
| Healthy Controls | 50.12±25.17 | |
| Clinical stage (n) AJCC criteria | Ia | 10 |
| Ib | 25 | |
| IIa | 10 | |
| IIb | 7 | |
| IIc | 5 | |
| III | 11 | |
| IV | 4 | |
| Disease spread (n) | localized | 57 |
| metastatic | 12 | |
| PFI (n) | <24 months | 22 |
| > 24 months | 48 | |
| TNM (n) | 1 | 35 |
| 2 | 22 | |
| 3 | 10 | |
| 4 | 5 | |
Samples
Venous blood was collected from melanoma patients in vacuettes with clot activator. After isolation (centrifugation at 3000 rpm for 10 minutes) serum samples were frozen at − 70 °C until testing (18).
Detection of IL-27
Concentration of IL-27 was measured by commercial ELISA kits (Human IL-27, DuoSet, R&D, Minneapolis, USA).
Statistical analysis
Data analysis was performed with Graph Pad Prism 5 software, using the nonparametric one-way ANOVA test with Bonferroni multiple comparison post-testing for analysis of multiple groups and Mann Whitney test for analysis of two groups.
Results
IL-27 values in melanoma patients groups according to the AJCC classification
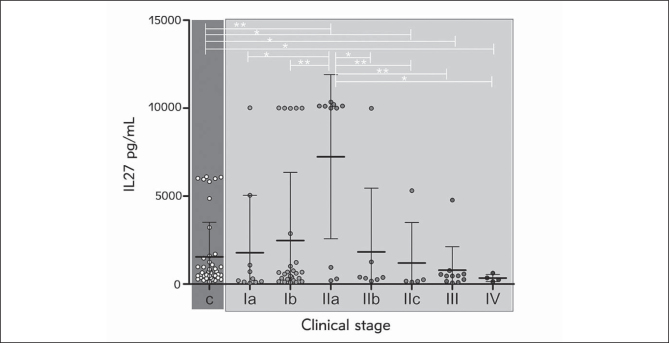
Average IL-27 concentration in melanoma patient samples showed a clear increment tendency from the earliest stage (Ia) up to maximal IL-27 levels in patients with stage IIa, followed by a decrement to the lowest average value in terminal disease stages, III and IV (Figure 1). Control samples contained significantly less IL-27 than those of melanoma patients with IIa (p=0.0064) stage but also significantly more IL-27 compared to patients with IIc (p=0.0281), III (p=0.0345) and IV (p=0.0351) AJCC stage. Finally, patients in IIa stage had significantly elevated IL-27 compared to Ia (p=0.0101), Ib (p=0.0095), IIb (p=0.033), IIc (p=0.0080), III (p=0.0067) and IV stage (p=0.0360).
Figure 1.
IL-27 values of melanoma patients grouped according to the AJCC classification. Values are expressed as mean ± SEM. *p < 0.05, **p < 0.01, c-controls.
IL-27 concentration in patients with localized and widespread disease
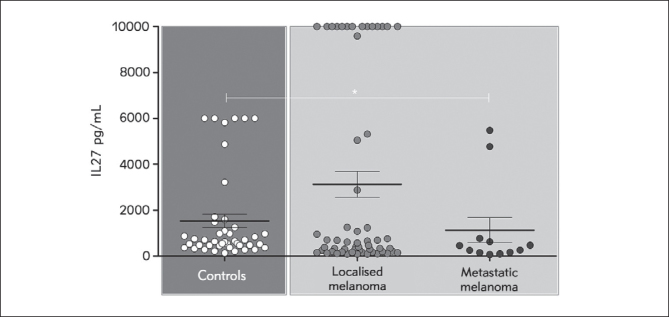
Patients with localized disease had insignificantly elevated average IL-27 concentrations compared to the group with metastatic disease (Figure 2). IL-27 concentration in the control group was significantly elevated compared to patients with metastatic disease (p=0.0259).
Figure 2.
IL-27 concentration in patients with localized and metastatic disease. Values are expressed as mean ± SEM. *p < 0.05, localized melanoma (I+II stage), metastatic melanoma (III+IV stage).
IL-27 values association with progression free interval (PFI)
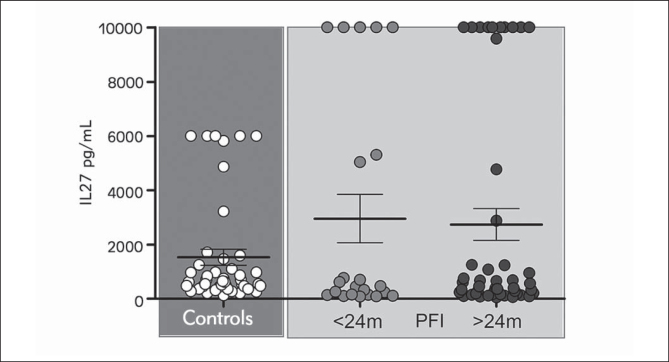
Patients who had a PFI shorter than 24 months had similar average IL-27 concentrations compared to the group with stable disease (Figure 3). Omission of patients with high IL-27 values (>1000 pg/mL) from both groups revealed an increased average concentration in the group with stable disease, but without statistical significance.
Figure 3.
IL-27 values association with progression free interval (PFI). Values are expressed as mean ± SEM. There was no statistical significance.
IL-27 values in melanoma patients grouped according to the TNM classification
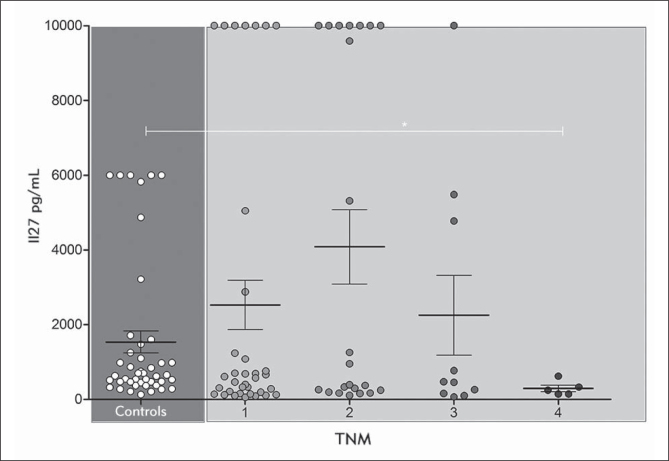
According to the TNM staging, the highest average IL-27 concentration was detected in patients with TNM2 (Figure 4). Patients with small melanomas (TNM1) as well as patients who had TNM stage 3 had similar IL-27 concentrations, while patients with large tumor and/or metastasis had the lowest average IL-27 value. Again, IL-27 concentration in the control group was significantly elevated compared to patients with metastatic disease.
Figure 4.
IL-27 values in melanoma patients according to TNM classification. Values are expressed as mean ± SEM. *p < 0.05
Association of clinical stage, disease spread, PFI and TNM status with frequency of melanoma patients with high IL-27
Analysis of IL-27 values according to the AJCC criteria classification demonstrated that most patients from IIa group had concentrations higher than 500 pg/mL (Table II). Identification of the so-called »high producers«, patients whose IL-27 sera levels were above 1000 and/or 5000 pg/mL, showed that they are highly frequent only in IIa group, while their frequency decreases below 25% in patients with more advanced disease (stage IIc–IV). Patients with PFI shorter or longer than 24 months did not differ in the number of persons with IL-27 sera concentration above 500 pg/mL. Contrary, high IL-27 producers with shorter PFI outnumbered patients who had longer periods of stable disease. Half of patients with TNM1 and TNM2 stage had IL-27 values over 500 pg/mL, with gradually decreasing frequency towards TNM4 stage. Advanced disease is inversely associated with the number of patients who had sera IL-27 above 500 pg/mL. Number of high IL-27 producers revealed a more pronounced ratio, with almoust twice higher frequency of those patients who had limited disease compared to patients who had advanced melanoma.
Table II.
Frequency of melanoma patients with IL-27 value above 500, 1000 and 5000 pg/mL
| Criteria | Stage | >500 pg/mL | >1000 pg/mL | >5000 pg/m L | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Ia | (4/10) | 40 | (3/10) | 30 | (2/10) | 20 | |
| AJCC | Ib | (14/25) | 56 | (8/25) | 32 | (6/25) | 24 |
| IIa | (8/10) | 80 | (7/10) | 70 | (7/10) | 70 | |
| IIb | (2/7) | 29 | (2/7) | 29 | (1/7) | 14 | |
| IIc | (1/5) | 20 | (1/5) | 20 | (1/5) | 20 | |
| III | (4/11) | 36 | (1/11) | 9 | (0/11) | 0 | |
| IV | (1/4) | 25 | (0/4) | 0 | (0/4) | 0 | |
| PFI | <24 | (10/22) | 45 | (7/22) | 32 | (7/22) | 32 |
| >24 | (23/48) | 48 | (16/48) | 33 | (11/48) | 23 | |
| TNM | 1 | (18/35) | 51 | (11/35) | 31 | (8/35) | 23 |
| 2 | (11/22) | 50 | (10/22) | 45 | (9/22) | 41 | |
| 3 | (4/10) | 40 | (3/10) | 30 | (2/10) | 20 | |
| 4 | (1/5) | 20 | (0/5) | 0 | (0/5) | 0 | |
| Disease | I+II | (29/57) | 51 | (21/57) | 37 | (17/57) | 30 |
| Spread | III+IV | (5/12) | 42 | (3/12) | 25 | (2/12) | 17 |
| Control | (29/44) | 66 | (13/44) | 30 | (6/44) | 14 | |
Discussion
Studies from Hisada and Salcedo were the first to report the antitumor effects of IL-27 (14, 16). The first report of antitumor activity of IL-27 in a murine model of colon carcinoma C26 from the Hisada group showed that IL-27 has potent abilities to induce tumor-specific antitumor activity and protective immunity which is mediated through CD8+T cells, IFN-γ and T-bet but not through STAT4 (14). Results from TBJ murine neuroblastoma tumors also demonstrated the ability of IL-27 to induce a tumor-specific protective immune response (16). In experimental studies, Shimizu et al. (17) showed strong inhibition of neovascularization by IL-27 in chicken embryos. They also showed that IL-27 has the ability to inhibit both tumor growth and metastasis of murine melanoma B16F10. Later, the Yoshimoto group (19) demonstrated in vitro potent antitumor effects of IL-27 on the poorly immunogenic melanoma cell line B16F10, mediated through NK cell activity. They also demonstrated that beyond antiproliferative activity, IL-27 had antiangiogenic activity, inducing expression of CCL9 and CXCL10. Authors concluded that IL-27 had antitumor effects on melanomas through WSX- 1/STAT1 signaling and proposed IL-27 as a potential antitumor therapeutic agent.
These data gave promising results that were waiting to be confirmed in studies with patients. There are still debates concerning the IL-27 function in cancer patients. It remains unclear whether high IL- 27 concentration in patient samples is associated with protective or insufficient antitumor response. Few studies reported correlation of high IL-27 concentration with limited disease or good response to therapeutical procedures. Study of the Diakowska group that was investigating IL-27 values in patients suffering from gastro-esophageal cancer (GEC) showed significant increase in IL-27 concentration in GEC patients compared to healthy controls and patients with benign diseases of the upper parts of digestive tract (20). Disease progression did not correlate significantly with IL-27 concentration. Our results showed an insignificant relationship between serum IL-27 and stage of the disease (TNM classification) with the highest average IL-27 level in patients with TNM2, while the patients at stage TNM4 had the lowest average values. Again, high IL-27 producers were the most numerous among patients with TNM2 stage. Study of patients with gastro-esophageal cancer showed significant correlation between serum IL- 27 level and lymph node status (N0, N1) and positive correlation between serum IL-27 and IFN-γ. IL-27 concentration did not differ significantly between groups of GEC patients with localized or disseminated disease. Although average IL-27 concentration and number of high IL-27 producers were higher in melanoma patients with localized disease, we did not establish a significant difference between patients with present or absent metastasis.
Another study showed increase in IL-27 together with VEGF concentration in patients with breast cancer compared to control subjects (21). IL-27 value was significantly associated with clinical stage and increased in the group of patients whose primary tumors were estrogen and progesterone receptor positive. The most important observation was that surgical treatment (modified radical mastectomy) was associated with IL-27 decrement. In patients with osteosarcoma, IL-27 concentration was significantly associated with tumor size, with increased concentration in those with tumors larger than 7 cm (22). Comparing the IL-27 value between groups with different histological types did not show any difference. Interestingly, in this study patients without metastasis had significantly increased IL-27 compared to those with disseminated disease, but patients with advanced clinical stage had significantly higher IL-27 compared to inital (III+IV vs I+II).
Two studies in lung cancer patients further highlighted the IL-27 importance. Naumnik et al. showed that IL-27 increment in broncho-alveolar fluid samples is associated with partial remission after chemotherapy (23). Duan et al. (24) found that NSCLC patients had significantly decreased average serum IL-27 concentrations compared to healthy controls (241 vs 320 pg/mL) and that IL-27 negatively correlated with blood Th17 cells percentage. Study on prostate cancer demonstrated the antitumor potential of IL-27 through inhibition of cell proliferation and by inducing apoptosis (25). Authors of this study showed that IL-27 downregulated pro-angiogenic genes such as vascular endothelial growth factor receptor (VEGFR)1/FLT1, fibroblast growth factor receptor 3 (FGFR3) and prostaglandin G/H synthase 1. IL-27 negatively regulates octeoclastogenesis and could even modulate interactions between prostate tumor and bone cells, which is important in treating metastatic prostate tumors (26).
Zhou et al. (27) demonstrated that patients with noninvasive bladder cancer had significantly decreased IL-27 compared to controls (21 vs 38 pg/mL). They found no significant differences in IL-27 concentration between invasive and noninvasive types of bladder cancer, nor between patients with high grade and low grade disease. Study by the Gonin group (28) demonstrated completely different data from the study of Zhou et al. (27). Analysis of IL-27 expression in primary cutaneous melanoma samples showed absence of IL-27 in benign pigment and noninvasive melanoma lesions, but intensive expression of IL-27 in primary invasive melanomas. In the IL-27 positive tumors, this cytokine was localized in macrophages, tumor infiltrating cells, melanophages, but also in tumor cells. Furthermore, patients with IL-27 positive tumors who suffered from disseminated disaese also had their metastasis positive for IL-27. Authors concluded that IL-27 expression in melanoma tissue was associated with disease progression, but not regression of tumor.
To our knowledge, this study is the first that investigated the IL-27 concentration in sera samples of melanoma patients. According to clinical staging, patients who were IIa stage had the highest average IL-27 level. Average IL-27 concentration had a clear decreasing trend toward patients with advanced disease. The frequency of high IL-27 producers also decreased together with progression of disease. The average value of IL-27 between groups PFI > 24 and PFI < 24 did not differ significantly, but the frequency of high IL-27 producers (IL-27 > 5000 pg/mL) was higher in the group with shorter PFI.
Taken together, the results of our study indicate a protective role of IL-27 in patients with melanoma. Understanding the role of IL-27 in the pathogenesis of melanoma could be useful for optimal control of the immune response against tumors. IL-27 could be valuable in the estimation of ongoing antitumor response.
Glossary
List of abbreviations
- AJCC
American Joint Committee on Cancer
- FGFR3
fibroblast growth factor receptor 3
- GEC
gastroesophageal cancer
- IL-27R
IL-27 receptor
- NK cells
natural killer
- NSCLC
non-small cell lung cancer
- PFI
progression free interval
- rpm
revolutions per minute
- TCCR
T cell cytokine receptor
- c
controls
Footnotes
Conflict of interest statement The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Bandarchi B, Ma L, Navab R, Seth A, Rasty G.. From melanocyte to metastatic malignant melanoma. Dermatol Res Pract 2010. 2010:583748. doi: 10.1155/2010/583748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scolyer RA, Long GV, Thompson JF.. Evolving concepts in melanoma classification and their relevance to multidisciplinary melanoma patient care. Mol Oncol. 2011;5(2):124–36. doi: 10.1016/j.molonc.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramirez-Montagut T, Turk MJ, Wolchok JD, Guevara-Patino JA, Houghton AN.. Immunity to melanoma: unraveling the relation of tumor immunity and autoimmunity. Oncogene. 2003;22(20):3180–7. doi: 10.1038/sj.onc.1206462. [DOI] [PubMed] [Google Scholar]
- 4.Ma MW, Medicherla RC, Qian M, Vega-Saenz de Miera E, Friedman EB, Berman RS. et al. Immune response in melanoma: an in-depth analysis of the primary tumor and corresponding sentinel lymph node. Mod Pathol. 2012;25(7):1000–10. doi: 10.1038/modpathol.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J. et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16(6):779–90. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 6.Wirtz S, Becker C, Fantini MC, Nieuwenhuis EE, Tubbe I, Galle PR. et al. EBV-induced gene 3 transcription is induced by TLR signaling in primary dendritic cells via NF-kappa B activation. J Immunol. 2005;174(5):2814–24. doi: 10.4049/jimmunol.174.5.2814. [DOI] [PubMed] [Google Scholar]
- 7.Larousserie F, Pflanz S, Coulomb-L’Herminé A, Brousse N, Kastelein R, Devergne O.. Expression of IL-27 in human Th1-associated granulomatous diseases. J Pathol. 2004;202(2):164–71. doi: 10.1002/path.1508. [DOI] [PubMed] [Google Scholar]
- 8.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF. et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172(4):2225–31. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 9.Batten M, Ghilardi N.. The biology and therapeutic potential of interleukin 27. J Mol Med (Berl) 2007;85(7):661–72. doi: 10.1007/s00109-007-0164-7. [DOI] [PubMed] [Google Scholar]
- 10.Villarino AV, Larkin J. Saris CJ, Caton AJ, Lucas S, Wong T. et al. Positive and negative regulation of the IL-27 receptor during lymphoid cell activation. J Immunol. 2005;174(12):7684–91. doi: 10.4049/jimmunol.174.12.7684. 3rd. [DOI] [PubMed] [Google Scholar]
- 11.Lucas S, Ghilardi N, Li J. de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100(25):15047–52. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamiya S, Owaki T, Morishima N, Fukai F, Mizuguchi J, Yoshimoto T.. An indispensable role for STAT1 in IL-27-induced T-bet expression but not proliferation of naive CD4+ T cells. J Immunol. 2004;173(6):3871–7. doi: 10.4049/jimmunol.173.6.3871. [DOI] [PubMed] [Google Scholar]
- 13.Owaki T, Asakawa M, Morishima N, Hata K, Fukai F, Matsui M. et al. A role for IL-27 in early regulation of Th1 differentiation. J Immunol. 2005;175(4):2191–200. doi: 10.4049/jimmunol.175.4.2191. [DOI] [PubMed] [Google Scholar]
- 14.Tulubus F, Mete R, Oznur M, Topcu B.. The role of adipocytokines in colon cancer and adenomas. J Med Biochem. 2014;33:135–42. [Google Scholar]
- 15.Chiyo M, Shimozato O, Yu L, Kawamura K, Iizasa T, Fujisawa T. et al. Expression of IL-27 in murine carcinoma cells produces antitumor effects and induces protective immunity in inoculated host animals. Int J Cancer. 2005;115(3):437–42. doi: 10.1002/ijc.20848. [DOI] [PubMed] [Google Scholar]
- 16.Salcedo R, Stauffer JK, Lincoln E, Back TC, Hixon JA, Hahn C. et al. IL-27 mediates complete regression of orthotopic primary and metastatic murine neuroblastoma tumors: role for CD8+ T cells. J Immunol. 2004;173(12):7170–82. doi: 10.4049/jimmunol.173.12.7170. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu M, Shimamura M, Owaki T, Asakawa M, Fujita K, Kudo M. et al. Antiangiogenic and antitumor activities of IL-27. J Immunol. 2006;176(12):7317–24. doi: 10.4049/jimmunol.176.12.7317. [DOI] [PubMed] [Google Scholar]
- 18.Lima-Oliveira G, Lippi G, Luca Salvagno G, Picheth G, Guidi CG.. Laboratory diagnostics and quality of blood collection. J Med Biochem. 2015;(34):288–94. doi: 10.2478/jomb-2014-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshimoto T, Morishima N, Mizoguchi I, Shimizu M, Nagai H, Oniki S. et al. Antiproliferative activity of IL27 on melanoma. J Immunol. 2008;180(10):6527–35. doi: 10.4049/jimmunol.180.10.6527. [DOI] [PubMed] [Google Scholar]
- 20.Diakowska D, Lewandowski A, Markocka-Maczka K, Grabowski K.. Concentration of serum interleukin-27 increase in patients with lymph node metastatic gastroesophageal cancer. Adv Clin Exp Med. 2013;22(5):683–91. [PubMed] [Google Scholar]
- 21.Lu D, Zhou X, Yao L, Liu C, Jin F, Wu Y.. Clinical implications of the interleukin 27 serum level in breast cancer. J Investig Med. 2014;62(3):627–31. doi: 10.2310/JIM.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 22.Tang YJ, Wang JL, Nong LG, Lan CG, Zha ZG, Liao PH.. Associations of IL-27 polymorphisms and serum IL-27p28 levels wiith osteosarcoma risk. Medicine. 2014;93(10):e56. doi: 10.1097/MD.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naumnik W, Naumnik B, Niewiarowska K, Ossolinska M, Chyczewska E.. Novel cytokines: IL-27, IL-29, IL-31 and IL-33. Can they be useful in clinical practice at the time diagnosis of lung cancer? Exp Oncol. 2012;34(4):348–53. [PubMed] [Google Scholar]
- 24.Duan M, Ning Z, Fu Z, Zhang J, Liu G, Wei Q. et al. Decreased IL-27 negatively correlated with Th17 cells in non-small cell lung cancer patients. Mediators Inflamm. 2015;2015:802939. doi: 10.1155/2015/802939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ðorđević D, Pejović J, Surbatović M, Jevđić J, Radaković S, Veljović M, Perić A, Anđelić T, Popović N.. Prognostic value and daily trend of interleukin-6, neutrophil cd64 expression, c-reactive protein and lipopolysaccharide-binding protein in critically ill patients: reliable predictors of outcome or not? J Med Biochem. 2015;(34):431–9. doi: 10.1515/jomb-2015-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zolochevska O, Diaz-Quiñones AO, Ellis J, Figueiredo ML.. Interleukin-27 expression modifies prostate cancer cell crosstalk with bone and immune cells in vitro. J Cell Physiol. 2013;228(5):1127–36. doi: 10.1002/jcp.24265. [DOI] [PubMed] [Google Scholar]
- 27.Zhou B, Zhang P, Tang T, Liao H, Zhang K, Pu Y. et al. Polymorphisms and plasma levels of IL-27: impact on genetic susceptibility and clinical outcome of bladder cancer. BMC Cancer. 2015(15):433. doi: 10.1186/s12885-015-1459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonin J, Carlotti A, Dietrich C, Audebourg A, Radenen-Bussière B, Caignard A. et al. Expression of IL-27 by tumor cells in invasive cutaneous and metastatic melanomas. PLoS One. 2013;8(10):e75694. doi: 10.1371/journal.pone.0075694. [DOI] [PMC free article] [PubMed] [Google Scholar]