Abstract
Objective
Transcatheter aortic valve implantation (TAVI) is widely used as an alternative to conventional surgical aortic valve replacement. The aim of this study was to identify preprocedural predictors of duration of length of stay (LoS) after transfemoral TAVI (TF-TAVI).
Methods
We included all consecutive patients who underwent TF-TAVI at our centre between November 2010 and June 2013. Preprocedural, periprocedural and postprocedural variables were collected and evaluated to LoS. Linear regression was performed to find preprocedural predictors for total LoS.
Results
The population consisted of 114 patients (mean age: 79.6±8.7, 32.5% male). The median total LoS was 6.5 days (5–9 days). Multivariate analysis showed that the Metabolic Equivalent score (METs) (β=−0.084, p=0.011) and diastolic blood pressure (β=−0.011, p=0.016) independently contributed to the log-transformed LoS.
Conclusion
Multivariate linear regression showed that lower METs and lower diastolic blood pressure were associated with prolonged LoS. Understanding patients’ physical functionality can improve logistical planning of hospital stay and selecting patients eligible for early discharge.
Keywords: transcatheter aortic valve implantation, length of stay, hospitalisation duration, predictors
Key questions.
What is already known about this subject?
Transcatheter aortic valve implantation (TAVI) is widely used as an alternative to conventional surgical aortic valve replacement. The aim of this study was to identify preprocedural predictors of postprocedural length of hospital stay after transfemoral TAVI. Current studies are merely focused on outcomes in terms of mortality and symptom relief. The TAVI minimalist approach is gaining popularity and should also include a reduction of length of stay and be timely.
What does this study add?
Our study describes our TAVI experience with the Edwards SAPIEN XT valve and preprocedural predictors for the duration of length of stay. Adding this knowledge to current literature allows better patient planning and selection of patients for early discharge. Furthermore it allows international comparison and finding best practices.
How might this impact on clinical practice?
In the upcoming years patient-tailored medicine is coming into practice and patient-tailored plans for admission and procedure are made. This study might help clinicians decide on forehand which patients can have an early discharge and allows them more patient-specific planning.
Introduction
Aortic valve replacement is indicated in case of severe aortic stenosis (AoS) or insufficiency.1 For patients with aortic valve stenosis, but at high risk for surgical aortic valve replacement, transcatheter aortic valve implantation (TAVI) is an appropriate alternative option.2 The transfemoral TAVI (TF-TAVI) approach is known to be the safest and therefore the most commonly used.3 4 Hospital length of stay (LoS) is associated with adverse events such as hospital-acquired infections and delirium.5 Postprocedural LoS may be influenced by various patient characteristics. Preprocedural risk assessment of LoS may yield several insights, such as appropriate patient information and individual organisational logistics.6 7 Additionally, some of the variables or patient characteristics may be optimised prior to the procedure to optimise the LoS. In this study we aim to elucidate preprocedural patient characteristics associated with LoS.
Methods
Patient population and procedure
This is a single-centre observational study with 114 consecutive patients undergoing TF-TAVI procedure between November 2010 and June 2013. All patients with severe symptomatic aortic valve stenosis were discussed by a multidisciplinary heart team and scheduled for a TF-TAVI procedure. TAVI procedures were performed according to standard techniques8 using the balloon expandable Edwards SAPIEN XT valve, in sizes of 23 mm, 26 mm and 29 mm. General anaesthesia was avoided, allowing immediate recognition of periprocedural complications such as a cerebrovascular accident, and possibly reducing the occurrence of delirium post procedure.9
Data collection and definitions
Data were collected retrospectively in a dedicated database and contained preprocedural, periprocedural and postprocedural variables. Preprocedural variables included demographic details that include medical history, symptoms, medication, blood pressure, laboratory values, ECG, transthoracic echocardiogram (TTE) and CT scan.
Standard surgical risk assessment was performed using the Society of Thoracic Surgeons (STS) score and the EuroSCORE (European System for Cardiac Operative Risk Evaluation).10 11 The Metabolic Equivalent score (METs) was measured using the Duke Activity Status Index and was used as an estimation of a patient’s functionality.12
Frailty was assessed by the Canadian Study of Health and Aging Clinical Frailty Score13 by means of the preoperative assessment by the anaesthesiology preprocedural screening regarding (non)instrumental activities and patient-reported daily life dependency. Medication before, during and after the procedure was also captured in our database. Preprocedural anticoagulants were categorised into five groups: (1) single antiplatelet drug, (2) dual antiplatelet therapy, (3) single oral anticoagulant, (4) single oral anticoagulant plus single antiplatelet drug or (5) single oral anticoagulant plus dual antiplatelet therapy. New oral anticoagulants and low-molecular weight heparin were labelled as oral anticoagulant.
Renal function, in terms of an estimated glomerular filtration rate (eGFR), was calculated using the modification of diet in renal disease (MDRD) formula.14
Preprocedural ECG assessment contained PQ time, QRS duration and QTc time.
Preprocedural TTE assessment encloses haemodynamic parameters, ejection fraction (EF%), other valve insufficiencies and systolic pulmonary artery pressure. Periprocedural variables included the duration of the procedure, selected valve size, amount of contrast media used and periprocedural success. Periprocedural success was defined as implantation of a single aortic valve in the correct position without any cardiovascular events or valve dysfunction within the first 72 hours.
Procedural time was calculated as the time between the patient’s arrival and discharge from operating room. All complications were analysed according to the Valve Academic Research Consortium (VARC) definitions.15 VARC two-criteria end points could not be used for this study as patient postprocedural urine output is not routinely measured, excluding the Acute Kidney Injury Network system and allowing only for the modified RIFLE (Risk, Injury, Failure, Loss of function, and End-stage kidney disease) classification.16 In addition to the VARC criteria, delirium, the need for a new pacemaker implantation and infections with a need for antibiotics were included as complications.
The hospital LoS was recorded as the total number of days between the TF-TAVI and the release of the patient from our centre.
Statistical analysis
Values are reported as mean±SD or median and IQR (IQR: 25th to 75th percentile) for continuous variables and as frequency with percentage for categorical variables. One-way analysis of variance and χ2 test for trends were used to compare the differences between groups of continuous and categorical variables, respectively. Group medians were compared using the Kruskal-Wallis test where appropriate. LoS was divided into three categories: (1) short stay (SS-LoS, 1–5 days), (2) medium stay (MS-LoS, 6–8 days) and (3) long stay (LS-LoS, 9+ days).
Covariates of interest as predictors of LoS were investigated using multivariable linear regression. Baseline variables that were significant at p≤0.10 on univariate analysis were entered into a multivariate model. All statistical tests were two-sided, and values of p≤0.05 were considered statistically significant. Statistical analysis was performed using SPSS V.22 for Windows (IBM Corp, New York, USA).
LoS was log-transformed to normalise the distribution prior to linear regression analysis.
Results
A total of 115 underwent TF-TAVI with the Edwards SAPIEN XT bioprosthesis between November 2010 and June 2013 at our centre. One patient died within hospital admission after developing acute kidney failure and was therefore excluded from the analysis, resulting in a study cohort of 114 patients. Baseline characteristics are shown in table 1. The average age was 79.6 (±8.7) years and 32.5% were male (n=37). Most patients had an American Society of Anesthesiologists score of III or IV (n=96, 86.5%). Local anaesthesia was used in 98% (n=112) of the cases.
Table 1.
Baseline characteristics
| Total population (N=114) | |
| Age (years) | 79.6±8.7 |
| Male, n (%) | 37 (32.5) |
| Body surface area (m²) | 1.88±0.21 |
| NYHA angina pectoris ≥ III, n (%) | 11 (9.6) |
| NYHA dyspnoea ≥ III, n (%) | 39 (34.2) |
| EuroSCORE 1 | 17.6±11.4 |
| Society of Thoracic Surgeons score | 6.6±5.6 |
| Estimated CSHA Clinical Frailty Score | 4.9±0.5 |
| ASA physical status classification system score (N=111) | |
| ASA I-II,n (%) | 15 (13.5) |
| ASA III-IV,n (%) | 96 (86.5) |
| Metabolic Equivalent score | 4 (3–6) |
| Risk factors | |
| Diabetes mellitus, n (%) | 30 (26.3) |
| Hypertension,n (%) | 74 (64.9) |
| Hyperlipidaemia, n (%) | 41 (36.0) |
| Positive family history, n (%) | 9 (7.9) |
| Medical history | |
| PCI, n (%) | 29 (25.4) |
| CABG, n (%) | 11 (9.6) |
| Pacemaker/ICD, n (%) | 12 (10.5) |
| Coronary artery disease, n (%) | 48 (42.1) |
| Myocardial infarction, n (%) | 15 (13.2) |
| Decompensated heart failure, n (%) | 36 (31.6) |
| Kidney failure, n (%) | 37 (32.5) |
| Peripheral vascular disease, n (%) | 16 (14.0) |
| CVA, n (%) | 7 (6.1) |
| TIA, n (%) | 23 (20.2) |
| Chronic lung disease, n (%) | 28 (24.6) |
| Liver cirrhosis, n (%) | 1 (0.9) |
| Medication | |
| Anticoagulant use, n (%) | 90 (78.9) |
| ACE inhibitor, n (%) | 37 (32.5) |
| Beta-blocker, n (%) | 62 (54.4) |
| Diuretic, n (%) | 77 (67.5) |
| Metformin, n (%) | 20 (17.5) |
| Statins, n (%) | 63 (55.3) |
Numbers given as mean±SD or, if not normally distributed, as median±IQR.
ASA, American Society of Anesthesiologists; CABG, coronary artery bypass grafting; CSHA, Canadian Study of Health and Aging; CVA, cerebrovascular accident; EuroSCORE, European System for Cardiac Operative Risk Evaluation; ICD, implantable cardioverter defibrillator; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; TIA, transient ischaemic attack.
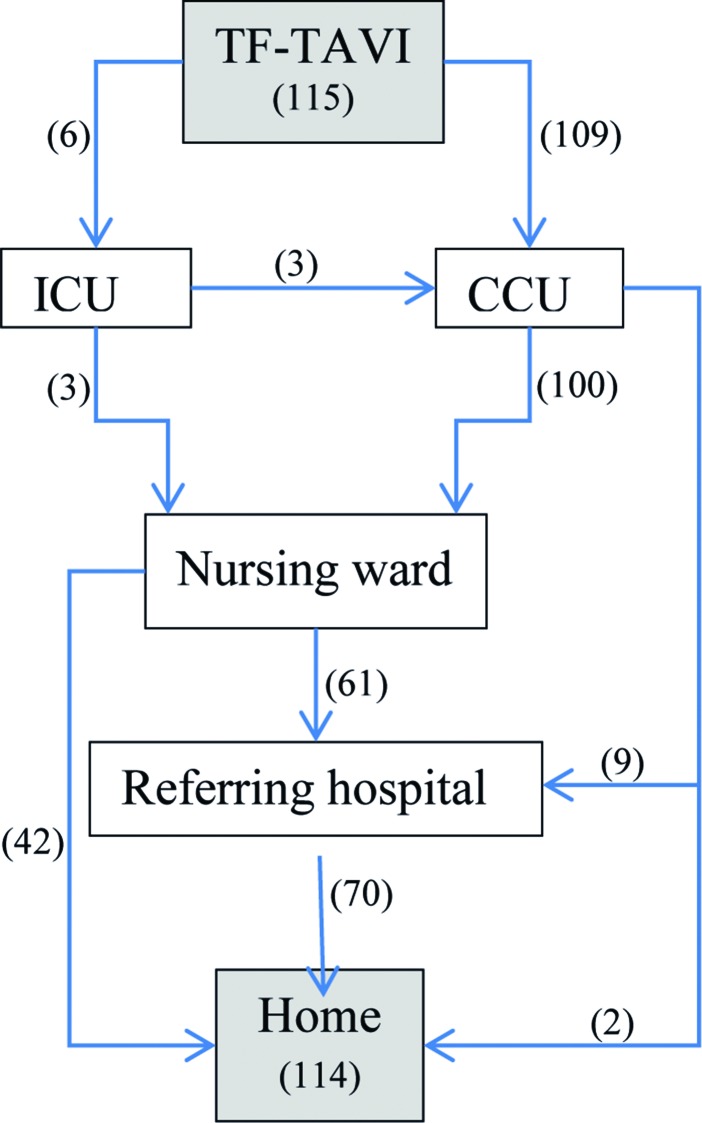
Figure 1 is a graphic representation of the hospital routing of the study cohort.
Figure 1.
Procedural flow chart. TF-TAVI, transfemoral transcatheter aortic valve implantation; ICU, intensive care unit; CCU, cardiac care unit.
The median total LoS was 6.5 days (IQR 5–9 days). The median LoS at the cardiac care unit was 1 day (IQR 1–1). Five patients were admitted at the intensive care unit, with a median of 2 days (IQR 1–7 days.)
Preprocedural variables
Preprocedural characteristics per grouped LoS are presented in table 2. There was a significant difference between LoS groups in age (p=0.004), STS-Risk of Procedural Mortality (STS-PROM) (p=0.007), Clinical Frailty Score (p=0.043), METs (p=0.004), diastolic blood pressure (p=0.023), N-terminal prohormone brain natriuretic peptide (p=0.038) and minimal diameter of the right iliac artery (p=0.026). A graphical representation of STS, EuroSCORE, METs and atrial fibrillation (AF) in the grouped LoS is shown in figure 2.
Table 2.
Preprocedural characteristics per grouped LoS
| Short stay (n=42) | Medium stay (n=43) | Long stay (n=29) | p Value grouped LoS | |
| Age (years) | 76.1±11.1 | 82.0±5.7 | 80.9±6.8 | 0.004 |
| Male, n (%) | 17 (41) | 14 (33) | 6 (21) | 0.084 |
| EuroSCORE 1 | 15.2±10.3 | 17.9±12.5 | 20.5±11.0 | 0.151 |
| STS-PROM | 4.5±3.1 | 7.4±5.9 | 8.4±7.1 | 0.007 |
| Clinical Frailty Score | 4.8±0.5 | 4.9±0.6 | 5.1±0.4 | 0.043 |
| METs | 5 (4.3–7.0) | 4 (3.0–5.0) | 4 (3.0–5.0) | 0.004 |
| Previous CVA, n (%) | 3 (7.1) | 4 (9.3) | 0 (0) | 0.257 |
| Previous chronic lung disease, n (%) | 12 (28.6) | 9 (20.9) | 7 (24.1) | 0.714 |
| Diabetes, n (%) | 13 (31.0) | 9 (20.9) | 8 (27.6) | 0.568 |
| Diastolic pressure (mm Hg) | 70.6±15.8 | 68.0±14.4 | 60.7±13.9 | 0.023 |
| Systolic pressure (mm Hg) | 142.8±30 | 142.6±26 | 133.2±23 | 0.270 |
| ECG | ||||
| Heart rate (beats/min) | 77.0±25.0 | 76.5±26.0 | 74.0±22.0 | 0.784 |
| Atrial fibrillation, n (%) | 5 (11.9) | 12 (27.9) | 11 (37.9) | 0.164 |
| PQ time (ms) | 168±37 | 172±60 | 192±48 | 0.825 |
| QTc time (ms) | 433±47 | 424±44 | 445±47 | 0.457 |
| QRS time (ms) | 102±28 | 92±26 | 99±18 | 0.806 |
| Conduction disorder, n (%) | 13 (31.0) | 17 (39.5) | 15 (51.7) | 0.100 |
| Echocardiogram | ||||
| Mean gradient (mm Hg) | 46±22 | 39±19 | 44±26 | 0.833 |
| Aortic valve area (cm²) | 0.78±0.23 | 0.79±0.22 | 0.74±0.30 | 0.697 |
| Aortic regurgitation (grades II–IV), n (%) |
10 (24.4) | 2 (4.7) | 7 (24.1) | 0.752 |
| LVF EF <30%, n (%) | 3 (7.1) | 2 (4.7) | 2 (7.0) | 0.402 |
| Mitral regurgitation (grades II–IV), n (%) |
17 (40.5) | 18 (41.9) | 21 (72.4) | 0.056 |
| Laboratory | ||||
| Haemoglobin | 8.0±1.1 | 7.7±1.1 | 7.6±0.8 | 0.349 |
| Haematocrit | 0.39±0.04 | 0.38±0.05 | 0.37±0.05 | 0.300 |
| INR | 1.02 (0.99–1.18) | 0.99 (0.97–1.16) | 1.06 (0.99–1.15) | 0.575 |
| Creatine | 97±63 | 102±70 | 100±47 | 0.944 |
| eGFR (MDRD) (mL/min/1.73 m2) | 70.0±24.7 | 64.1±24.5 | 60.6±11.8 | 0.243 |
| NT-proBNP | 1001 (618–2233) | 1512 (823–3040) | 1869 (1011–4139) | 0.038 |
| CT parameters | ||||
| Annulus diameter (mm) | 23.8±2.1 | 23.7±1.5 | 22.6±2.2 | 0.161 |
| Minimal diameter iliac R (mm) | 7.9±1.3 | 7.8±1.3 | 6.9±1.4 | 0.026 |
| Minimal diameter iliac L (mm) | 7.9±1.5 | 7.9±1.1 | 7.2±1.7 | 0.230 |
CVA, cerebrovascular accident; EF, ejection fraction; eGFR, estimated glomerular filtration rate; EuroSCORE, European System for Cardiac Risk Evaluation; INR, international normalised ratio; LoS, length of stay; LVF, left ventricular function; MDRD, modification of diet in renal disease; METs, Metabolic Equivalent score; NT-proBNP, N-terminal prohormone brain natriuretic peptide; STS-PROM, Society of Thoracic Surgeons-Risk of Procedural Mortality.
Figure 2.
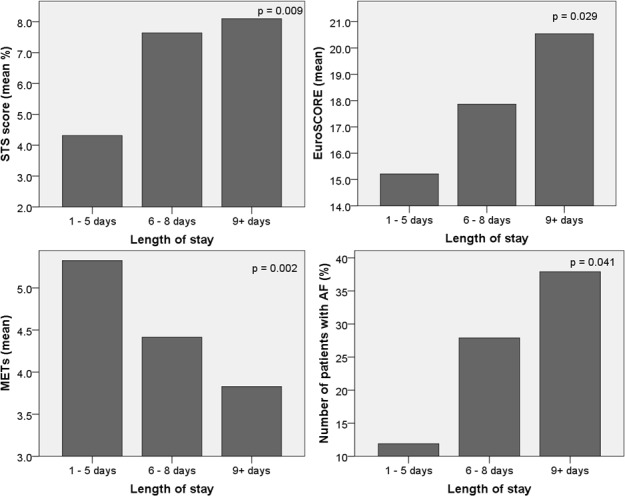
Figures showing the significant correlations between increased length of stay and increased STS scores, increased EuroSCORE, higher METs and pre-existent AF. AF, atrial fibrillation; EuroSCORE, European System for Cardiac Operative Risk Evaluation; METs, Metabolic Equivalent score; STS, Society of Thoracic Surgeons.
Univariate and subsequent multivariate analyses showed that there was a significant association with LoS for STS score (p<0.001), baseline diastolic blood pressure (p=0.013) and METs (p=0.003).
Univariate analysis on preprocedural variables (table 3) showed that STS score (β=0.037, p<0.001), baseline diastolic blood pressure (β=−0.010, p=0.013), METs (β=−0.086, p=0.003) and atrial fibrillation (β=0.277, p=0.041) are associated with LoS.
Table 3.
Univariate and multivariate linear regression analyses: variables of table 2 showing a significance of p<0.10 were taken in the multivariate model
| Univariate | Multivariate | |||
| Beta | p Value | Beta | p Value | |
| Age (years) | 0.013 | 0.054 | ||
| EuroSCORE 1 logistic | 0.011 | 0.029 | ||
| STS | 0.037 | <0.001 | ||
| METs | −0.086 | 0.003 | −0.084 | 0.011 |
| Diastolic pressure (mm Hg) | −0.010 | 0.013 | −0.011 | 0.016 |
| Atrial fibrillation | 0.277 | 0.041 | ||
| Mitral regurgitation (II–IV) | 0.116 | 0.056 | ||
| Minimal right iliac diameter | −0.109 | 0.012 | ||
| Laboratory | ||||
| Haemoglobin | −0.111 | 0.055 | ||
| eGFR (MDRD) (mL/min/1.73 m2) | −0.004 | 0.077 | ||
| NT-proBNP | 0.00006 | 0.054 |
Length of stay is log-transformed to normalise the distribution.
eGFR, estimated glomerular filtration rate; EuroSCORE, European System for Cardiac Operative Risk Evaluation; MDRD, modification of diet in renal disease; METs, metabolic equivalent score; NT-proBNP, N-terminal prohormone brain natriuretic peptide; STS, Society of Thoracic Surgeons.
ECG characteristics such as QRS duration, QT time and conduction disorders (left bundle branch block (LBBB), right bundle branch block (RBBB), intraventricular conduction disorders (IVCD), atrioventricular block (AVB)) did not show a significant association with LoS.
Periprocedural variables
As expected, complications were related to a longer LoS. Periprocedural complications are shown in table 4. The median LoS is described for the various complications. A higher LoS can be expected after a periprocedural myocardial infarction, a major bleeding, acute kidney injury, minor vascular complication and hospital-acquired infection.
Table 4.
Postprocedural complications and their correlation with the total length of stay in days
| Complication | No of patients (N=114), n (%) |
Median days (IQR) |
| Overall | 114 | 6.5 (5–9) |
| No complications | 52 (45.6) | 6 (4–8) |
| Temporary pacemaker wire not removed during procedure | 56 (49.1) | 6.5 (5–11.75) |
| Complications | ||
| Periprocedural myocardial infarction | 2 (1.7) | 13.50 (5–22) |
| Spontaneous myocardial infarction | 0 | - |
| TIA | 0 | - |
| Minor stroke | 2 (1.7) | 6.50 (5–8) |
| Major stroke | 0 | - |
| Life-threatening bleeding | 0 | - |
| Major bleeding | 2 (1.8) | 17 (6-28) |
| Minor bleeding | 22 (19.3) | 6 (4.5–11.5) |
| AKI stage I | 11 (9.6) | 12 (7-22) |
| AKI stage II | 2 (1.7) | 13.50 (5-22) |
| AKI stage III | 0 | - |
| Major vascular complications | 0 (0.0) | - |
| Minor vascular complications | 20 (17.5) | 7 (5–11) |
| Hospital-related infection | 9 (7.8) | 8 (6.5–17.5) |
| Pacemaker implantation | 5 (4.4) | 6.5 (4.5–46.75) |
| Delirium | 5 (4.4) | 7 (4–25) |
AKI, acute kidney injury; TIA, transient ischaemic attack; VARC, Valve Academic Research Consortium.
The difference between removing and remaining the temporary external pacemaker wire before leaving the cathlab did not differ significantly between the LoS groups (p=0.419). The implantation of a permanent pacemaker did not lead to a longer median LoS.
Outcomes in terms of mortality are described in table 5.
Table 5.
Outcomes for study cohort
| Mortality | No of patients (N=114) |
| 30-Day cardiovascular mortality | 1 (0.9 %) |
| All-cause mortality 6 months | 7 (6.1%) |
| All-cause mortality 1 year | 10 (8.8%) |
| All-cause mortality 3 years | 29 (25.4%) |
Discussion
In this retrospective study we found two predictors of post-TF-TAVI LoS. In a multivariate model, only diastolic blood pressure (negative association) and METs remained significant.
Risk scores
In agreement with O’Brien et al 10 and Toumpoulis et al 11 an increase in STS-PROM score and EuroSCORE, respectively, was significantly associated with LoS. For the TAVI population the EuroSCORE is mainly used to predict operative mortality, however the Toumpoulis et al study also showed in a total cohort of 5051 cardiac surgery patients (of which 285 were aortic valve surgery patients) the correlation between the EuroSCORE and an LoS of ≥12 days. In our cohort we did not find a significant relation between EuroSCORE and LoS, whereas STS score was significantly different. However, there was a trend of higher risk scores in the longer LoS groups.
Both the STS score and EuroSCORE are primarily made up of components that cannot be altered or optimised prior to the procedure, therefore acting solely as predictor, and not as an opportunity to decrease the LoS.
As the STS-PROM showed a greater significance with LoS, it would be advisable to use this score when looking to predict LoS. In a study by Arangalage et al 17 EuroSCORE II was shown to be more similar to the STS-PROM score than the original EuroSCORE.
Frailty
The significance between METs and the trend with the estimated Clinical Frailty Score show that patients’ disabilities, physical functioning and social network play a role in LoS. The estimated Clinical Frailty Score puts its emphasis more on being able to independently complete activities of daily living (ADL) or instrumental ADL, whereas METs looks specifically at the amount of energy used to complete these tasks. The Duke activity status index, which was used to estimate the METs, does reflect the level of physical functioning. Both these scores, however, are subjectively determined and therefore both only serve as an indication for patients’ capacities. If medical urgency allows, actions to possibly improve METs or Clinical Frailty Score, such as improving strength and balance using physical therapy, could be offered prior to the procedure if patients’ symptoms allow it. A parallel path would be to look into making alterations at home to improve possibilities of mobility and functionality at home, thereby making it possible for patients to further recover at home, rather than in the hospital. If in further research these variables are proven to shorten LoS, a physiotherapist or specialised nurse may be indicated as an important addition to the heart team discussions and planning for patients. As this would still be a subjective approach to predicting LoS, an altered validated frailty risk score should be developed to objectify the frailty and risk of patients prior to the procedure. As the STS score only looks at the chronological age of patients, an incorporation of the biological age might be beneficial.18
Age itself was not a predictor for LoS in the linear regression model. This might be that calendar age itself does not reflect the level of independence and level of physical activity, which is reflected by METs.
Kidney function
The influence of the kidney function on LoS has also been made apparent by means of eGFR. Furthermore creatine levels show a trend with LoS.
As kidney function seems to be a key predictor, it may be advisable to standardly use the newer CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula instead of the MDRD in the everyday clinical setting. This formula uses the same four variables as the MDRD formula (creatine level, age, gender and race) but uses a formula that has been shown to be more accurate in estimating the glomerular filtration rate (GFR), especially at higher GFRs.19
A common aetiology of a decreased kidney function is likely to be multifactorial with the populations’ increased biological age, atherosclerosis, hypertension and medication usage. In this study the age of patients showed a trend with a higher LoS, which corresponds with many other studies, such as by Malaisrie et al.20
Medication
Preprocedural anticoagulant use was stopped prior to TAVI except for acetylsalicylic acid and/or P2Y12 inhibitors. The categorised combinations of preprocedural anticoagulant medication were significant with the total LoS; however, it was not significant with any individual complications that would be suspected from anticoagulant use.
Chronic diuretic use was the only preprocedural medication that had a trend with LoS.
Cardiac factors
Extra emphasis on the management of AF pharmaceutically prior to the procedure may be an option to decrease LoS, ss 25 patients with AF were found to be using beta-blockers, despite its relative contraindication in significant AoS. Further research is needed to improve the available treatment for AF and to test AF as a risk factor.
The development of new conduction disorders or the need for a permanent pacemaker implantation was not associated with LoS, which is surprising, but might be explained by the low number of events (n=5/114).
As many patients suffer from the combination of aortic stenosis and regurgitation, it is difficult to isolate the cause for the significance of the diastolic blood pressure and the trend with patients also suffering from mitral regurgitation. Diastolic blood pressure was negatively associated with LoS and can be associated with an increase in stiffness of the arterial wall in older patients with atherosclerosis or chronic kidney disease. The pathophysiology in relation to the LoS is interesting and could be part of future research.
The measurements taken from CT angiography (minimal diameters of the left and right iliac arteries) may allow for a reconsideration of equipment used during the procedure. The current generation of Edwards SAPIEN 3 valves can be placed using a 14 Fr catheter, which already has been shown to reduce complications.21 Using angiography may give a more precise diameter of the annulus and the iliac arteries, and could be considered to improve accuracy.22
Complications
As predicted, many of the complications correlate significantly with LoS, especially the major complications. The need for transfusions was associated with longer LoS and may reflect a weaker patient status. The influence of the acute kidney injury (stage 1) on LoS shows again the importance of managing kidney function.
In our population, most of the infections were urinary tract infections occurring within 3 days of the procedure; however, there were two cases of infections that occurred after 11 and 29 days. These may have been prevented by an earlier discharge from the hospital.
Limitations and future directions
Despite the relatively small sample size of this study, it provides insight in the first years of TAVI on a larger scale, allowing future comparisons. Second, to provide optimal care for patients, it is important to find a balance between early and delayed discharge. To better understand the consequences of early discharge, it is important to also take into account rehospitalisation and care provided by (informal) caregivers. A third limitation is the inclusion of Edwards SAPIEN XT valves only. We recommend to include in subsequent studies newer valve types of various manufacturers.
Preprocedural aortic and mitral insufficiencies were not identically assessed excluding the parameters from this study.
Conclusions
Prolonged LoS was associated with higher values of STS-PROM and EuroSCORE I. Even so for lower values of eGFR, METs, CT-based right femoral diameter and diastolic blood pressure. Finally, the presence of pre-existing AF was associated with prolonged LoS as shown by univariate analysis. After multivariate linear regression METs was the most important variable that remained. METs (estimated with the Duke activity status index) is an easy-to-measure variable in daily clinical practice. Understanding patients’ physical functionality can improve logistical planning of hospital stay and selecting patients eligible for early discharge.
Footnotes
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Questions regarding data sharing can be adressed to the corresponding author.
References
- 1. Nishimura RA, Otto CM, Bonow RO, et al. AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Thorac Cardiovasc Surg 2014;2014:e1–e132. [DOI] [PubMed] [Google Scholar]
- 2. Kirtane AJ, Leon MB. The placement of aortic transcatheter valve (PARTNER) trial: clinical trialist perspective. Circulation 2012;125:3229–32. 10.1161/CIRCULATIONAHA.112.093070 [DOI] [PubMed] [Google Scholar]
- 3. Svensson LG, Blackstone EH, Rajeswaran J, et al. ; PARTNER Trial Investigators. Comprehensive analysis of mortality among patients undergoing TAVR: results of the PARTNER trial. J Am Coll Cardiol 2014;64:158–68. 10.1016/j.jacc.2013.08.1666 [DOI] [PubMed] [Google Scholar]
- 4. Petronio AS, Capranzano P, Barbato E, et al. Current status of transcatheter valve therapy in Europe: results from an EAPCI survey. EuroIntervention 2016;12:890–5. 10.4244/EIJY16M06_01 [DOI] [PubMed] [Google Scholar]
- 5. George AJ, Boehme AK, Siegler JE, et al. Hospital-acquired infection underlies poor functional outcome in patients with prolonged length of stay. ISRN Stroke 2013;2013:1–5. 10.1155/2013/312348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chevreul K, Brunn M, Cadier B, et al. ; FRANCE Registry Investigators. Cost of transcatheter aortic valve implantation and factors associated with higher hospital stay cost in patients of the FRANCE (FRench aortic national CoreValve and Edwards) registry. Arch Cardiovasc Dis 2013;106:209–19. 10.1016/j.acvd.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 7. Siebens K, Miljoen H, Fieuws S, et al. Implementation of the guidelines for the management of patients with chest pain through a critical pathway approach improves length of stay and patient satisfaction but not anxiety. Crit Pathw Cardiol 2010;9:30–4. 10.1097/HPC.0b013e3181d24549 [DOI] [PubMed] [Google Scholar]
- 8. Webb JG, Pasupati S, Humphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007;116:755–63. 10.1161/CIRCULATIONAHA.107.698258 [DOI] [PubMed] [Google Scholar]
- 9. Wiegerinck EM, Boerlage-van Dijk K, Koch KT, et al. Towards minimally invasiveness: transcatheter aortic valve implantation under local analgesia exclusively. Int J Cardiol 2014;176:1050–2. 10.1016/j.ijcard.2014.07.170 [DOI] [PubMed] [Google Scholar]
- 10. O'Brien SM, Shahian DM, Filardo G, et al. ; Society of Thoracic Surgeons Quality Measurement Task Force. The society of thoracic surgeons 2008 cardiac surgery risk models: part 2--isolated valve surgery. Ann Thorac Surg 2009;88:S23–42. 10.1016/j.athoracsur.2009.05.056 [DOI] [PubMed] [Google Scholar]
- 11. Toumpoulis IK, Anagnostopoulos CE, Swistel DG, et al. Does EuroSCORE predict length of stay and specific postoperative complications after cardiac surgery?. Eur J Cardiothorac Surg 2005;27:128–33. 10.1016/j.ejcts.2004.09.020 [DOI] [PubMed] [Google Scholar]
- 12. Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke activity status index). Am J Cardiol 1989;64:651–4. 10.1016/0002-9149(89)90496-7 [DOI] [PubMed] [Google Scholar]
- 13. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–95. 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levey AS, Coresh J, Greene T, et al. ; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–54. 10.7326/0003-4819-145-4-200608150-00004 [DOI] [PubMed] [Google Scholar]
- 15. Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol 2011;57:253–69. 10.1016/j.jacc.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 16. Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012;33:2403–18. 10.1093/eurheartj/ehs255 [DOI] [PubMed] [Google Scholar]
- 17. Arangalage D, Cimadevilla C, Alkhoder S, et al. Agreement between the new EuroSCORE II, the logistic EuroSCORE and the society of thoracic surgeons score: implications for transcatheter aortic valve implantation. Arch Cardiovasc Dis 2014;107:353–60. 10.1016/j.acvd.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 18. Green P, Arnold SV, Cohen DJ, et al. Relation of frailty to outcomes after transcatheter aortic valve replacement (from the PARTNER trial). Am J Cardiol 2015;116:264–9. 10.1016/j.amjcard.2015.03.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stevens LA, Schmid CH, Greene T, et al. Comparative performance of the CKD epidemiology collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis 2010;56:486–95. 10.1053/j.ajkd.2010.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malaisrie SC, McCarthy PM, McGee EC, et al. Contemporary perioperative results of isolated aortic valve replacement for aortic stenosis. Ann Thorac Surg 2010;89:751–6. 10.1016/j.athoracsur.2009.11.024 [DOI] [PubMed] [Google Scholar]
- 21. Ando T, Briasoulis A, Holmes AA, et al. Sapien 3 versus sapien XT prosthetic valves in transcatheter aortic valve implantation: a meta-analysis. Int J Cardiol 2016;220:472–8. 10.1016/j.ijcard.2016.06.159 [DOI] [PubMed] [Google Scholar]
- 22. Wiegerinck EM, Marquering HA, Oldenburger NY, et al. Imaging for approach selection of TAVI: assessment of the aorto-iliac tract diameter by computed tomography-angiography versus projection angiography. Int J Cardiovasc Imaging 2014;30:399–405. 10.1007/s10554-013-0343-2 [DOI] [PubMed] [Google Scholar]