Fig. 3.
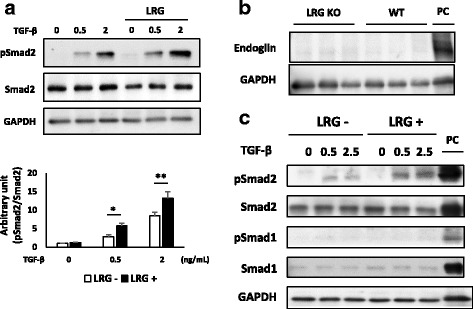
Recombinant leucine-rich alpha 2 glycoprotein (LRG) enhances transforming growth factor beta (TGF-β)-induced Smad2 phosphorylation in naïve CD4 T cells and promotes T regulatory cell differentiation. a Naïve CD4 T cells were isolated from LRG knockout (LRG KO) mice and were stimulated by TGF-β (0.5 or 2 ng/mL) in the presence or absence of recombinant LRG (LRG + (1 μg/mL) or LRG -) for 30 minutes. Representative data from three independent experiments are shown (upper panel). The levels of phosphorylated Smad2 relative to total Smad2 were quantified using imageJ and values (mean ± SD; n =3) are shown (bottom panel): *p < 0.05, **p < 0.01, one-way analysis of variance followed by the Bonferroni test. NT non-treated. b Naïve CD4 T cells were isolated from wild-type (WT) or LRG KO mice (n = 3, respectively). The expression of endoglin was determined by western blotting. MS-1, mouse endothelial cell, was used as a positive control (PC) for the expression of endoglin. c Naïve CD4 T cells were obtained from LRG KO mice and stimulated by TGF-β (0, 0.5 and 2.5 ng/mL) in the absence or presence of recombinant LRG (LRG + (1 μg/mL) or LRG -) for 30 minutes. The phosphorylation of Smad1 and Smad2 were examined by western blotting. L929, mouse fibroblast, was used as a PC. GAPDH glyceraldehyde-3-phosphate dehydrogenase
