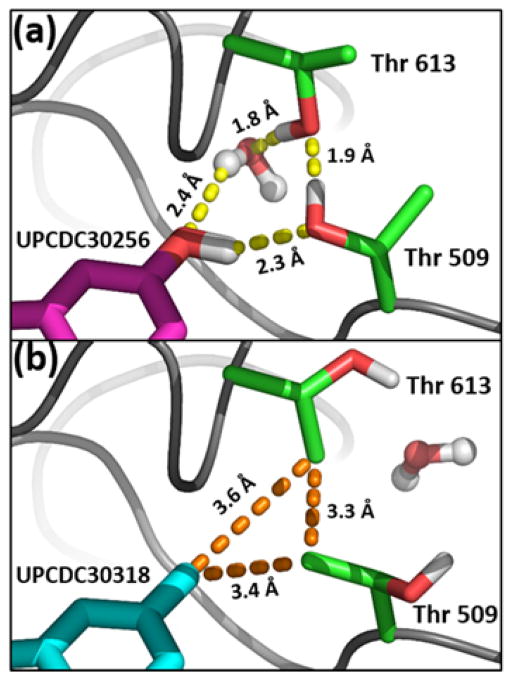
Fig. 3.
Upon inhibitor binding, the loops containing the Thr 509-Thr 613 pair collapse to form an amphiphilic binding site at the indole 5-position. (a) Polar subsite: the Thr γ-hydroxyls engage in a network of hydrogen bonds with polar inhibitor substituents. (b) Hydrophobic subsite: rotation of the Thr χ1 torsions by 150–180° results in γmethyl group interactions with small hydrophobic moieties on the indole 5-position. In this conformation, the bridging water molecule has been displaced from the binding pocket.