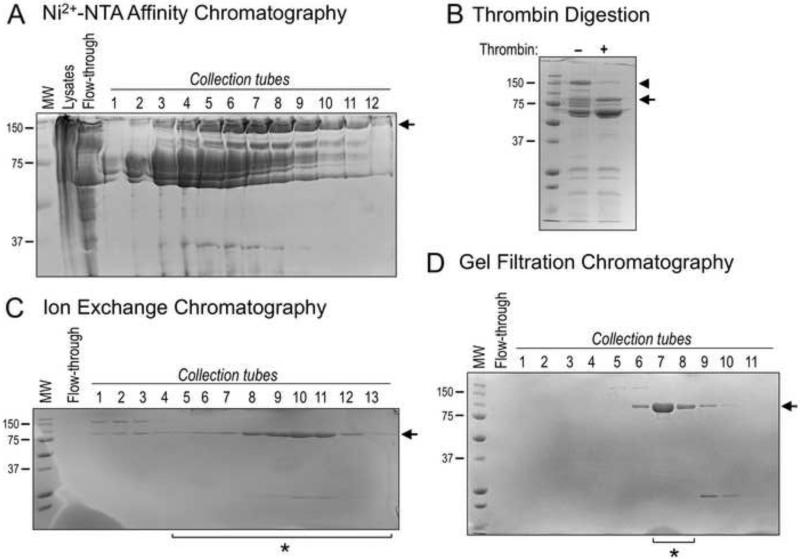
Figure 2. Purification of Recombinant MicalredoxCH protein.
(A) Coomassie-stained bands (arrow) corresponding to the Nus-tagged MicalredoxCH protein were observed in multiple collection tubes following IPTG-induced bacterial expression, lysis, Ni2+-NTA affinity chromatography, and elution with 250 mM imidazole. MW in kDa (also for B-D).
(B) Samples from (A) containing Ni2+-NTA affinity purified Nus-tagged MicalredoxCH protein were combined and subjected to thrombin digestion (+) to remove the Nus tag. A Coomassie-stained band corresponding in size to the cleaved MicalredoxCH protein was observed following thrombin digestion (arrow). Note, that uncleaved Nus-tagged MicalredoxCH protein is readily seen in the absence of thrombin (arrowhead).
(C) As seen following Coomassie-staining, Cation exchange chromatography was used to separate MicalredoxCH protein (arrow) from contaminating proteins, including chaperonins and the Nus tag. Proteins were eluted with a gradient of NaCl by increasing the percentage of S-B buffer. Sample tubes 5 through 13 (*) were utilized for (D).
(D) Gel filtration chromatography allowed the further purification of the MicalredoxCH protein as seen following Coomassie-staining such that the protein collected in tubes 7 and 8 (*) was combined, concentrated to a working volume, and utilized for the results presented in Figures 3 and 4. We find an overall yield of ~2 mg of highly-pure active MicalredoxCH protein can be routinely generated from 6 L of bacterial culture using this purification strategy and the starting culture volume can be scaled-up in order to purify larger amounts of MicalredoxCH protein.