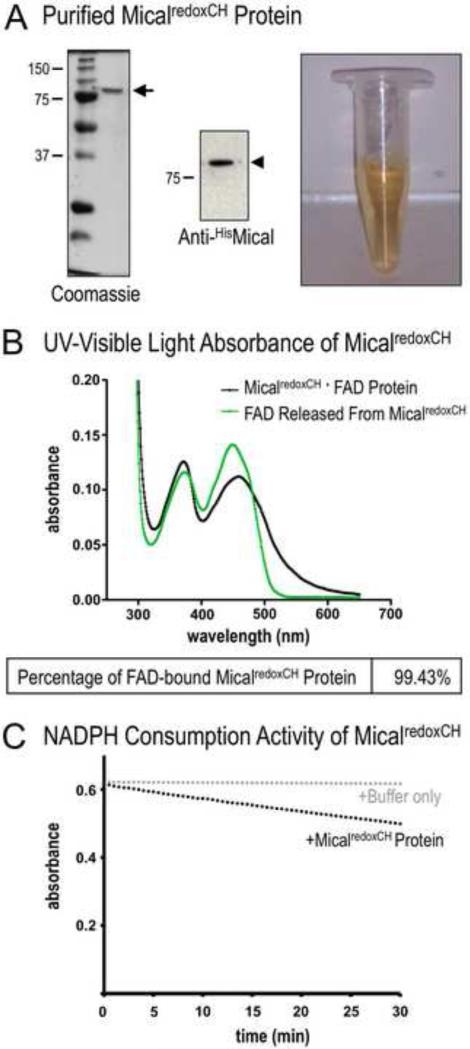
Figure 3. Determining the purity of the MicalredoxCH protein.
(A) Purified Mical protein in the presence of its prosthetic group (co-factor) FAD. SDS-PAGE/Coomassie-staining (left) and anti-His immunoblotting (middle) reveal both the presence of His-tagged MicalredoxCH protein (arrowhead) and the purity of the protein sample (arrow). Note also that this purified Mical protein is yellow in color (right). In particular, active Mical protein requires FAD to be purified along with the Mical protein backbone and FAD generates a yellowish color that can be used to confirm the presence of FAD. MW in kDa.
(B) The isoalloxazine ring system of FAD that generates the yellow/orange color of FAD (see Figure 3A) is also responsible for light absorption in the UV and visible spectral range such that the oxidized form of FAD has two peaks at ~360nm and ~450nm [19] (green line). Note, that the UV-visible light absorption spectra of the purified MicalredoxCH protein sample also reveal peaks at ~360 nm and ~450 nm (black line, peaks at 372nm and 459nm). This characteristic absorption spectra is a hallmark of a flavin binding protein. Characterization of the absorption spectra also allows a determination of the amount of the purified MicalredoxCH that is bound to FAD. The concentration of purified Mical in this sample is 4.2 mg/ml (50.16 μM) (see Materials and Methods). The concentration of FAD in this sample is 49.87 μM (0.4487/8998 M−1cm−1) (see Materials and Methods). Thus, 99.43% (49.87 μM/50.16 μM) of MicalredoxCH protein in the sample is bound to FAD.
(C) We confirmed the enzymatic activity of purified MicalredoxCH protein by following the conversion of Mical's co-enzyme NADPH to NADP+. This conversion (consumption) of NADPH can be followed by measuring the change in absorbance at 340 nm (NADPH absorbs light at 340 nm, while NADP+ does not). Note the change (decrease) in absorbance of NADPH over time in the presence of MicalredoxCH protein (black dots) but not with buffer only (the buffer used to store MicalredoxCH; gray dots). Consistent with our previous results [11], adding Mical's substrate, F-actin, into this assay further increases (in a F-actin concentration-dependent manner) the consumption of NADPH by MicalredoxCH protein (not shown).