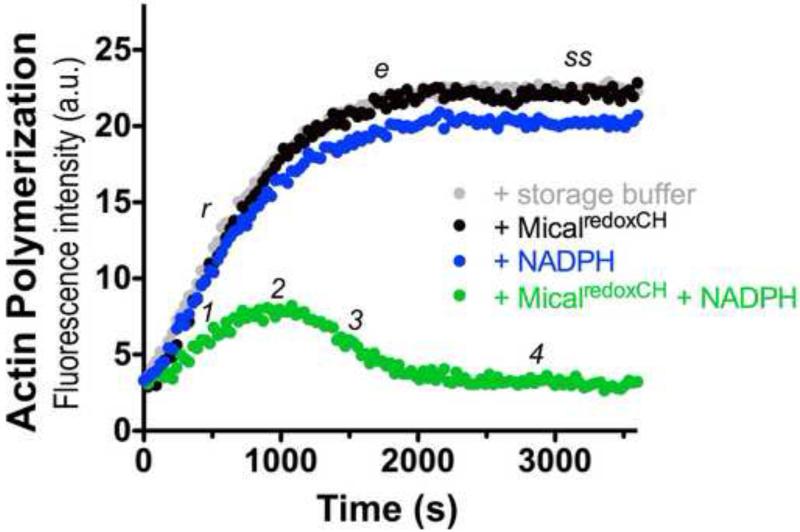
Figure 4. Analysis of the activity of purified MicalredoxCH protein.
Pyrene-labeled actin was used to monitor both the polymerization and depolymerization of actin, where the fluorescence intensity (a.u. [arbitrary units]) of the pyrene-labeled actin polymer is substantially higher than the pyrene-labeled actin monomer. As judged by the increase in fluorescence intensity over time which reveals actin polymerization, the addition of 600 nM of purified MicalredoxCH protein alone to actin (black dots) does not alter the rate (r), extent (e), or steady-state level (ss) of actin polymerization (as compared to an actin only control incubated with the buffer used to store MicalredoxCH; gray dots). In the presence of 100 μM of its NADPH coenzyme, however, purified MicalredoxCH protein (MicalredoxCH + NADPH; green dots) specifically alters the rate of actin polymerization (1) such that over time activated MicalredoxCH induces a decrease in the extent of polymerization (2), the rapid depolymerization of F-actin (3), and the inability of actin to reinitiate polymer formation (4). These results are similar to what we observed with our previously purified active MicalredoxCH enzyme [10-12].