Introduction
Negative symptom pathology in patients with schizophrenia is an unmet therapeutic need. Beyond schizophrenia, however, amotivation, alogia, and affective flattening are also observed in several other disorders (see Strauss and Cohen, this issue1). Given that negative symptoms, together with impaired cognition, predict functional outcome in schizophrenia and depression patients,2–4 the development of effective treatment strategies is urgently required. Developing targeted treatments requires a greater understanding of the mechanisms underlying such symptoms. This understanding requires objective quantification of negative symptom features that can be applied in animal models. Recent reviews provided insights on techniques and targets to be investigated,5–8 including a review by Green and colleagues7 that culminated in a drive toward understanding effort-based decision making. The potential that delineating the mechanisms underlying effort-based decision making could provide novel treatment targets is an important direction for the field. The impact of this research and their resulting assessment of mechanisms underlying negative symptoms of patients with schizophrenia and other psychiatric conditions using preclinical models are discussed below.
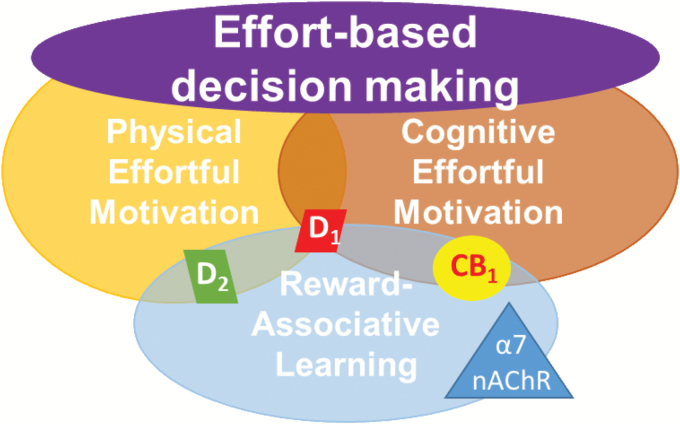
This novel direction toward quantifying effort-based decision making is being increasingly reflected in preclinical research, including parsing contributions to such decision making. Early rodent-based investigations of schizophrenia-related negative symptoms focused on depression-relevant tests such as sucrose/saccharin preference tests,9–12 but evidence suggests patients with schizophrenia exhibit normal sweet solution preference despite their high negative symptom scores.13 In contrast, people with schizophrenia have consistently exhibited poor reward-associative behaviors (eg, probabilistic learning14,15), when rewards are explicitly linked to outcome. Deficits in probabilistic learning are also evident in patients with major depression. In contrast to patients with schizophrenia, however, whose deficits are linked to reductions in reward sensitivity, patients with depression display a greater sensitivity to misleading negative feedback.16 Similarly, implicit reward-associative learning deficits, which can be measured across species,5,17 is seen in other psychiatric conditions, such as depression and bipolar disorder,18,19 but not schizophrenia.20,21 Such reward-associative learning forms one aspect of effort-based decision making, wherein subjects weigh the benefit of an outcome (ie, reward) against the costs required to obtain it (ie, effort expenditure). Increased physical effort to obtain a reward can be assessed by measuring whether an animal scales a surmountable barrier or presses a lever multiple times to obtain a desirable reward (eg, 4 sucrose pellets), as opposed to opting for the less desirable alternative reward (eg, 2 sucrose pellets) by not expending additional effort.22,23 Willingness to engage in more cognitively demanding behavior to receive a desirable reward can be assessed by letting the animal choose between trials that present long or short visual stimuli that must be accurately detected.24 The short visual stimulus is more difficult to detect and is, therefore, the more cognitively demanding choice. An abnormal inflation of perceived effort (cognitive or physical) to obtain a reward, or a reduced perceived value of a reward, would affect a subject’s willingness to engage in such effortful behavior. Hence, deficits in the ability to accurately compute effort/cost may translate into motivational impairments evident in schizophrenia patients. Effort-based decision making is therefore subserved by domains including: physical effortful motivation25; cognitive effortful motivation; and reward associative learning (reviewed in6, see figure 1). Delineating the neural mechanisms subserving these domains, and their commonalities and differences, may result in novel treatment targets that could be individually tailored to the patient.
Fig. 1.
Subdomains contributing to effort-based decision making. Effort-based decision making is a critical component of negative symptoms in schizophrenia and can be readily assessed in humans and rodents. Further, subdomains are identified whose neural mechanisms can be investigated in rodent studies. Dopamine D1 receptors have been implicated in each aspect of decision making, while dopamine D2 receptors contribute toward physical effort and reward-associative learning. Both cannabinoid CB1 and α7 nicotinic acetylcholine receptors have been implicated in reward associative learning, while CB1 receptors may also drive cognitive effortful motivation.
Rodent studies investigating neural mechanisms of physical and cognitive effort have begun given the elevated perceived cost of cognitive effort observed in patients,26–29 ultimately leading to reduced overall effort.30,31 Both physical and cognitive effortful motivation were able to predict global cognitive scores (outcome) in schizophrenia patients.27 Interestingly, in rodents both physical and cognitive effortful discounting were reduced by perturbation of the prefrontal cortex (PFC),32,33 similar to behavioral deficits and observations of altered PFC functioning in schizophrenia patients. Furthermore, cannabis treatment reduced effortful choices and reduced response-bias development in humans.34 Similarly, treatment with the psychoactive ingredient of cannabis, tetrahydrocannabinoid (THC), also reduced cognitive effort.35 Hence, the endocannabinoid system—an on-demand system only activated when required—could be involved in the overall reduced motivation of patients with schizophrenia. It is also important to note, however, that these 2 domains are also dissociable because dopamine receptor antagonists reduce physical but not cognitive effort.33 The dopamine system is also important for reward learning, whereby adeno-associated viral dopamine D1 receptor suppression in the striatum impaired probabilistic learning, but not effortful breakpoint in mice.36 Similarly, infusion of the dopamine D1 receptor antagonist SCH23390, although not the D2 antagonist eticlopride, into the anterior cingulate cortex (ACC) disrupted effort-cost decision making.37 In contrast, systemic dopamine D2-like receptor antagonism impaired such effort-based decision making that was remediated with a systemic dopamine D1-like receptor antagonist.38 These systemic effects may be mediated via the nucleus accumbens because dopamine D2 receptor over-expression in this region increased the willingness to expend effort, confirming the role of ventral striatal dopamine transmission in motivational processing.39 In contrast, however, D2 receptor over-expression in the striatum reduced the willingness to expend effort for a preferred reward in mice.40,41 Given that positive symptoms of schizophrenia patients are treated with dopamine D2-like receptor antagonists, their effort-cost decision making deficits may be exaggerated, and this dopaminergic interaction remains complicated. Indirect action on dopamine receptors may provide alternative targets for remediation of negative symptoms.42 For example, α7 nicotinic acetylcholine receptor (nAChR) activation releases dopamine43 that preferentially acts on dopamine D1 receptors,44 perhaps explaining why mice lacking these receptors exhibit some depression-relevant behaviors including immobility in the forced swim test and reduced sucrose preference45 and impaired probabilistic learning, although they exhibit normal effortful behavior.46 Interestingly, some clinical trials indicated that α7 nAChR agonist treatments reduced negative symptoms of patients with schizophrenia.47–49 Thus, its contribution to reward learning may be important for treating negative symptoms, but large-scale follow-up trials are required with negative symptoms as the primary target. Hence, alterations to the endocannabinoid, nicotinic, and/or dopaminergic systems likely contribute to negative motivational states in schizophrenia. More studies are required, however, to test these mechanisms, their locations of effect, and whether they are involved during schizophrenia-relevant manipulations (see below). Moreover, additional studies are required to specifically investigate potential schizophrenia-related pathophysiology in these domains.
The impact of schizophrenia-relevant manipulations on motivational behaviors has also been investigated. For example, the maternal immune activation model (wherein early developmental immune activation) of schizophrenia resulted in an elevated breakpoint in a progressive ratio breakpoint task.50 This finding is in direct contrast with schizophrenia patients who exhibit reduced breakpoints,25 but the authors maintained that the elevated breakpoint may have been related to an inability to detect changes in reward/behavior contingencies, leading to perseverative-like behaviors.50 The increased breakpoint is similar, however, to our recent studies demonstrating that repeated phencyclidine treatment (a commonly used manipulation for modeling schizophrenia), also increased breakpoint in rats, even after a 2-week washout period. In contrast, isolation rearing-induced deficits were observed in the probabilistic reversal learning task, although these deficits were not observed when tested in a single session,51 as is done in clinical populations.15 Hence, manipulations commonly used to model cognitive deficits of schizophrenia patients do not always recreate schizophrenia-relevant effort-based decision making.
Some psychiatric disorder-relevant manipulations have resulted in negative symptom-related behaviors, such as reducing Sp4 expression in mice from birth (which negatively impacts numerous systems including NMDA receptor expression52), reduced physical effort and lowered reward-associative learning.53 Environmental manipulations also induce depression-relevant behaviors such as social defeat disrupting implicit reward associative learning in rats.54 This profile was associated with alterations in stress-related peptide mRNA in the striatum and decreased activity in the ventral tegmental area. Environmental stress also induces negative affective changes in the affective bias task in rats,55 which may be useful in future studies across multiple psychiatric conditions. Other relevant manipulations arise from the observation that psychiatric disorder diagnoses are higher in births during spring months, leading to the postulation that reduced vitamin D during development may have negative outcomes.56 Indeed, developmental vitamin D (DVD) deficient rats exhibit some schizophrenia-like behaviors.57 In contrast, however, DVD rats exhibited normal risk-preference in a rat gambling task,58 unlike schizophrenia patients whom exhibit deficient Iowa Gambling Task performance that are linked to negative symptoms.59 To-date, few investigations of negative symptom-related behaviors have been examined using this developmental inducing condition. Given that DVD deficient-induced behaviors have not always been comparable across rats and mice,60,61 nor within strains of mice,62 other mechanisms may drive spring birth-induced schizophrenia-like behaviors.
Given the nature of this commentary, not covered here is a comprehensive overview of other important topics. Full descriptions of the tasks described have not been provided but if interested, the reader is directed toward the appropriate references. One point that should be made clear irrespective of task though is that researchers control for any potential indirect effects of treatments or manipulations, eg, slowed motoric capability, perseverative behavior, cognitive impairments etc. that may contribute to disruptions in task performance. Exhaustively discussing these necessary controls is beyond the scope of this short commentary, but these are important considerations and are often performed within the same task (eg, blocking both arms in the T-maze with barriers or measuring response/reward latencies in operant tasks) or by conducting complementary, multivariate assessment of behavior (eg, locomotor activity/open-field or a battery of cognitive testing) to aid the interpretation of a specific change in effortful decision making. Other areas not covered are the use/utility of social-based tasks to assess negative symptom-related behavioral profiles in animal models. Although there is increasing use of such tasks,63,64 their links to negative symptoms remain unclear, as do their specificity to negative vs cognitive deficits of psychiatric patients. Similarly, studies have begun using rodent ultrasonic vocalizations.65–67 Such discussions are beyond the scope of this short commentary, but were reviewed in part by Wilson and Koenig.68 Another avenue of future investigations is the observation that cognitive remediation can attenuate negative symptom scores in schizophrenia patients.69 Delineating the neural mechanisms of its effect could be useful for developing more targeted therapeutics, and/or for validating animal models of schizophrenia.
The recent work outlined above (summarized in table 1) provides quantifiable targets of disordered behavior in schizophrenia patients linked to their negative symptoms. The advent of modern techniques in neuroscience that allows unparalleled visualization and/or manipulation of neural activity during the assessment of reward-related behaviors is advancing our fundamental understanding of how the brain processes reward-related stimuli.70,72 Combining these modern techniques with behavioral procedures that assess reward with high translational validity, and inducing conditions that impair effort-based decision making in a manner consistent with those observed in disorders characterized by negative symptoms, may lead to a better mechanistic understanding of this cluster of symptoms and the development of future treatments for individuals with negative symptoms behavioral profiles.
Table 1.
Summary of Studies on Mechanisms and Models Underlying Effort-Based Decision Making Related Negative Symptoms (PFC, Prefrontal Cortex; ACC, Anterior Cingulate Cortex; THC, Tetrahydrocannabinoid; GlyT, Glycine 1 Transporter)
| Study Type | Domain | Manipulation | Brain Region | Effect on Behavior | Ref. |
|---|---|---|---|---|---|
| Mechanistic | Cost/benefit decision making | GABAa antagonist | PFC | Impaired decision making | 32 |
| Physical/ cognitive effort | Dopamine D1- or D2-family receptor antagonists | Systemic | Decreased physical effort, little effect on cognitive effort | 33 | |
| Cognitive effort | THC/CB1 receptor agonist | Systemic | Decreased cognitive effort | 35 | |
| Reward learning | Suppression of dopamine D1 receptor expression | Striatum | Impaired probabilistic learning; effort unaffected | 36 | |
| Physical effort | Dopamine D1-family receptor antagonist | ACC | Impaired physical effort | 37 | |
| Physical effort | Dopamine D2-family receptor antagonist | Systemic | Impaired physical effort | 38 | |
| Physical effort | Dopamine D2 receptor overexpression | Nucleus accumbens | Increased physical effort expenditure | 39 | |
| Physical effort | Dopamine D2 receptor overexpression | striatum | Impaired physical effort | 40,41 | |
| Physical effort | GlyT1 inhibition | N/A | No effect | 52 | |
| Animal Models of Negative Symptoms | Reward learning | Knockout of α7 nicotinic acetylcholine receptors | N/A | Impaired probabilistic learning | 43 |
| Physical effort | Maternal immune activation | N/A | Increased breakpoints | 50 | |
| Physical effort | Repeated phencyclidine | N/A | Increased breakpoint | ||
| Reward learning | Social Isolation Rearing | N/A | Impaired probabilistic reversal learning | 51 | |
| Physical effort | Reduced Sp4 expression | N/A | Reduced physical effort | 53 | |
| Reward learning | Reduced Sp4 expression | N/A | Impaired reward associative learning | 53 | |
| Reward learning | Social defeat | Striatum, VTA | Blunted response bias | 54 |
Funding
This work was funded in part by National Institute of Mental Health grant funding (R01MH104344 and R01MH108653); a NARSAD Young Investigator Award; as well as the Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center.
Acknowledgments
The authors would like to highlight the importance of Dr. Athina Markou on the development of each of our careers, a giant in the field, sorely missed. The authors report no conflict of interest.
References
- 1. Strauss GP, Cohen AS. A transdiagnoostic review of negative symptom phenomonology and etiology. Schizophr Bull. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rassovsky Y, Horan WP, Lee J, Sergi MJ, Green MF. Pathways between early visual processing and functional outcome in schizophrenia. Psychol Med. 2011;41:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr Bull. 2006;32:259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch Gen Psychiatry. 2012;69:1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Der-Avakian A, D’Souza MS, Pizzagalli DA, Markou A. Assessment of reward responsiveness in the response bias probabilistic reward task in rats: implications for cross-species translational research. Transl Psychiatry. 2013;3:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Young JW, Markou A. Translational rodent paradigms to investigate neuromechanisms underlying behaviors relevant to amotivation and altered reward processing in schizophrenia. Schizophr Bull. 2015;41:1024–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Green MF, Horan WP. Effort-based decision making in schizophrenia: evaluation of paradigms to measure motivational deficits. Schizophr Bull. 2015;41:1021–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barnes SA, Der-Avakian A, Markou A. Anhedonia, avolition, and anticipatory deficits: assessments in animals with relevance to the negative symptoms of schizophrenia. Eur Neuropsychopharmacol. 2014;24:744–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Distler MG, Opal MD, Dulawa SC, Palmer AA. Assessment of behaviors modeling aspects of schizophrenia in Csmd1 mutant mice. PLoS One. 2012;7:e51235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barkus C, Feyder M, Graybeal C et al. Do GluA1 knockout mice exhibit behavioral abnormalities relevant to the negative or cognitive symptoms of schizophrenia and schizoaffective disorder? Neuropharmacology. 2012;62:1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vardigan JD, Huszar SL, McNaughton CH, Hutson PH, Uslaner JM. MK-801 produces a deficit in sucrose preference that is reversed by clozapine, D-serine, and the metabotropic glutamate 5 receptor positive allosteric modulator CDPPB: relevance to negative symptoms associated with schizophrenia? Pharmacol Biochem Behav. 2010;95:223–229. [DOI] [PubMed] [Google Scholar]
- 12. Karlsson RM, Tanaka K, Saksida LM, Bussey TJ, Heilig M, Holmes A. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2009;34:1578–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berlin I, Givry-Steiner L, Lecrubier Y, Puech AJ. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. Eur Psychiatry. 1998;13:303–309. [DOI] [PubMed] [Google Scholar]
- 14. Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 2007;62:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reddy LF, Waltz JA, Green MF, Wynn JK, Horan WP. Probabilistic reversal learning in schizophrenia: stability of deficits and potential causal mechanisms. Schizophr Bull. 2016;42:942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taylor Tavares JV, Clark L, Furey ML, Williams GB, Sahakian BJ, Drevets WC. Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage. 2008;42:1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pergadia ML, Der-Avakian A, D’Souza MS et al. Association between nicotine withdrawal and reward responsiveness in humans and rats. JAMA Psychiatry. 2014;71:1238–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pizzagalli DA, Goetz E, Ostacher M, Iosifescu DV, Perlis RH. Euthymic patients with bipolar disorder show decreased reward learning in a probabilistic reward task. Biol Psychiatry. 2008;64:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahnallen CG, Liverant GI, Gregor KL et al. The relationship between reward-based learning and nicotine dependence in smokers with schizophrenia. Psychiatry Res. 2012;196:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heerey EA, Bell-Warren KR, Gold JM. Decision-making impairments in the context of intact reward sensitivity in schizophrenia. Biol Psychiatry. 2008;64:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Floresco SB, Ghods-Sharifi S. Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cereb Cortex. 2007;17:251–260. [DOI] [PubMed] [Google Scholar]
- 23. Bryce CA, Floresco SB. Perturbations in effort-related decision-making driven by acute stress and corticotropin-releasing factor. Neuropsychopharmacology. 2016;41:2147–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cocker PJ, Hosking JG, Benoit J, Winstanley CA. Sensitivity to cognitive effort mediates psychostimulant effects on a novel rodent cost/benefit decision-making task. Neuropsychopharmacology. 2012;37:1825–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wolf DH, Satterthwaite TD, Kantrowitz JJ et al. Amotivation in schizophrenia: integrated assessment with behavioral, clinical, and imaging measures. Schizophr Bull. 2014;40:1328–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Culbreth A, Westbrook A, Barch D. Negative symptoms are associated with an increased subjective cost of cognitive effort. J Abnorm Psychol. 2016;125:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Strauss GP, Morra LF, Sullivan SK, Gold JM. The role of low cognitive effort and negative symptoms in neuropsychological impairment in schizophrenia. Neuropsychology. 2015;29:282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gold JM, Kool W, Botvinick MM, Hubzin L, August S, Waltz JA. Cognitive effort avoidance and detection in people with schizophrenia. Cogn Affect Behav Neurosci. 2015;15:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Granholm E, Verney SP, Perivoliotis D, Miura T. Effortful cognitive resource allocation and negative symptom severity in chronic schizophrenia. Schizophr Bull. 2007;33:831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol. 2012;121:553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bowie CR, Milanovic M, Tran T, Cassidy S. Disengagement from tasks as a function of cognitive load and depressive symptom severity. Cogn Neuropsychiatry. 2017;22:83–94. [DOI] [PubMed] [Google Scholar]
- 32. Piantadosi PT, Khayambashi S, Schluter MG, Kutarna A, Floresco SB. Perturbations in reward-related decision-making induced by reduced prefrontal cortical GABA transmission: relevance for psychiatric disorders. Neuropharmacology. 2016;101:279–290. [DOI] [PubMed] [Google Scholar]
- 33. Hosking JG, Floresco SB, Winstanley CA. Dopamine antagonism decreases willingness to expend physical, but not cognitive, effort: a comparison of two rodent cost/benefit decision-making tasks. Neuropsychopharmacology. 2015;40:1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lawn W, Freeman TP, Pope RA et al. Acute and chronic effects of cannabinoids on effort-related decision-making and reward learning: an evaluation of the cannabis ‘amotivational’ hypotheses. Psychopharmacology (Berl). 2016;233:3537–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Silveira MM, Adams WK, Morena M, Hill MN, Winstanley CA. Δ(9)-Tetrahydrocannabinol decreases willingness to exert cognitive effort in male rats. J Psychiatry Neurosci. 2017;42:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Higa KK, Young JW, Ji B, Nichols DE, Geyer MA, Zhou X. Striatal dopamine D1 receptor suppression impairs reward-associative learning. Behav Brain Res. 2017;323:100–110. [DOI] [PubMed] [Google Scholar]
- 37. Schweimer J, Hauber W. Dopamine D1 receptors in the anterior cingulate cortex regulate effort-based decision making. Learn Mem. 2006;13:777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yohn SE, Santerre JL, Nunes EJ et al. The role of dopamine D1 receptor transmission in effort-related choice behavior: Effects of D1 agonists. Pharmacol Biochem Behav. 2015;135:217–226. [DOI] [PubMed] [Google Scholar]
- 39. Trifilieff P, Feng B, Urizar E et al. Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Mol Psychiatry. 2013;18:1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li YC, Kellendonk C, Simpson EH, Kandel ER, Gao WJ. D2 receptor overexpression in the striatum leads to a deficit in inhibitory transmission and dopamine sensitivity in mouse prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108:12107–12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ward RD, Simpson EH, Richards VL et al. Dissociation of hedonic reaction to reward and incentive motivation in an animal model of the negative symptoms of schizophrenia. Neuropsychopharmacology. 2012;37:1699–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Acheson DT, Twamley EW, Young JW. Reward learning as a potential target for pharmacological augmentation of cognitive remediation for schizophrenia: a roadmap for preclinical development. Front Neurosci. 2013;7:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wonnacott S, Barik J, Dickinson J, Jones IW. Nicotinic receptors modulate transmitter cross talk in the CNS: nicotinic modulation of transmitters. J Mol Neurosci. 2006;30:137–140. [DOI] [PubMed] [Google Scholar]
- 44. Hamada M, Higashi H, Nairn AC, Greengard P, Nishi A. Differential regulation of dopamine D1 and D2 signaling by nicotine in neostriatal neurons. J Neurochem. 2004;90:1094–1103. [DOI] [PubMed] [Google Scholar]
- 45. Zhang JC, Yao W, Ren Q et al. Depression-like phenotype by deletion of α7 nicotinic acetylcholine receptor: role of BDNF-TrkB in nucleus accumbens. Sci Rep. 2016;6:36705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Young JW, Meves JM, Tarantino IS, Caldwell S, Geyer MA. Delayed procedural learning in α7-nicotinic acetylcholine receptor knockout mice. Genes Brain Behav. 2011;10:720–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Umbricht D, Keefe RS, Murray S et al. A randomized, placebo-controlled study investigating the nicotinic α7 agonist, RG3487, for cognitive deficits in schizophrenia. Neuropsychopharmacology. 2014;39:1568–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lieberman JA, Dunbar G, Segreti AC et al. A randomized exploratory trial of an α-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38:968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Young JW, Geyer MA. Evaluating the role of the alpha-7 nicotinic acetylcholine receptor in the pathophysiology and treatment of schizophrenia. Biochem Pharmacol. 2013;86:1122–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Millar J, Bilkey DK, Ward RD. Maternal immune activation alters sensitivity to action-outcome contingency in adult rat offspring. Brain Behav Immun. 2017;63:81–87. [DOI] [PubMed] [Google Scholar]
- 51. Amitai N, Young JW, Higa K, Sharp RF, Geyer MA, Powell SB. Isolation rearing effects on probabilistic learning and cognitive flexibility in rats. Cogn Affect Behav Neurosci. 2014;14:388–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou X, Nie Z, Roberts A et al. Reduced NMDAR1 expression in the Sp4 hypomorphic mouse may contribute to endophenotypes of human psychiatric disorders. Hum Mol Genet. 2010;19:3797–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Young JW, Kamenski ME, Higa KK, Light GA, Geyer MA, Zhou X. GlyT-1 inhibition attenuates attentional but not learning or motivational deficits of the Sp4 hypomorphic mouse model relevant to psychiatric disorders. Neuropsychopharmacology. 2015;40:2715–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Der-Avakian A, D’Souza MS, Potter DN et al. Social defeat disrupts reward learning and potentiates striatal nociceptin/orphanin FQ mRNA in rats. Psychopharmacology (Berl). 2017;234:1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stuart SA, Butler P, Munafò MR, Nutt DJ, Robinson ES. A translational rodent assay of affective biases in depression and antidepressant therapy. Neuropsychopharmacology. 2013;38:1625–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cope ZA, Powell SB, Young JW. Modeling neurodevelopmental cognitive deficits in tasks with cross-species translational validity. Genes Brain Behav. 2016;15:27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McGrath JJ, Burne TH, Féron F, Mackay-Sim A, Eyles DW. Developmental vitamin D deficiency and risk of schizophrenia: a 10-year update. Schizophr Bull. 2010;36:1073–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Peak JN, Turner KM, Burne TH. The effect of developmental vitamin D deficiency in male and female Sprague-Dawley rats on decision-making using a rodent gambling task. Physiol Behav. 2015;138:319–324. [DOI] [PubMed] [Google Scholar]
- 59. Martino DJ, Bucay D, Butman JT, Allegri RF. Neuropsychological frontal impairments and negative symptoms in schizophrenia. Psychiatry Res. 2007;152:121–128. [DOI] [PubMed] [Google Scholar]
- 60. Turner KM, Young JW, McGrath JJ, Eyles DW, Burne TH. Cognitive performance and response inhibition in developmentally vitamin D (DVD)-deficient rats. Behav Brain Res. 2013;242:47–53. [DOI] [PubMed] [Google Scholar]
- 61. Harms LR, Turner KM, Eyles DW, Young JW, McGrath JJ, Burne TH. Attentional processing in C57BL/6J mice exposed to developmental vitamin D deficiency. PLoS One. 2012;7:e35896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Harms LR, Eyles DW, McGrath JJ, Mackay-Sim A, Burne TH. Developmental vitamin D deficiency alters adult behaviour in 129/SvJ and C57BL/6J mice. Behav Brain Res. 2008;187:343–350. [DOI] [PubMed] [Google Scholar]
- 63. Schneider S, Götz K, Birchmeier C, Schwegler H, Roskoden T. Neuregulin-1 mutant mice indicate motor and sensory deficits, indeed few references for schizophrenia endophenotype model. Behav Brain Res. 2017;322:177–185. [DOI] [PubMed] [Google Scholar]
- 64. Watson DJ, King MV, Gyertyán I, Kiss B, Adham N, Fone KC. The dopamine D₃-preferring D₂/D₃ dopamine receptor partial agonist, cariprazine, reverses behavioural changes in a rat neurodevelopmental model for schizophrenia. Eur Neuropsychopharmacol. 2016;26:208–224. [DOI] [PubMed] [Google Scholar]
- 65. Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J. Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping, lesion, and pharmacology studies. Behav Brain Res. 2007;182:274–283. [DOI] [PubMed] [Google Scholar]
- 66. Swalve N, Mulholland MM, Schulz TD, Li M. Effects of the phencyclidine model of schizophrenia and nicotine on total and categorized ultrasonic vocalizations in rats. Behav Pharmacol. 2016;27:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Peters SM, Tuffnell JA, Pinter IJ, van der Harst JE, Spruijt BM. Short- and long-term behavioral analysis of social interaction, ultrasonic vocalizations and social motivation in a chronic phencyclidine model. Behav Brain Res. 2017;325(Pt A):34–43. [DOI] [PubMed] [Google Scholar]
- 68. Wilson CA, Koenig JI. Social interaction and social withdrawal in rodents as readouts for investigating the negative symptoms of schizophrenia. Eur Neuropsychopharmacol. 2014;24: 759–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cella M, Preti A, Edwards C, Dow T, Wykes T. Cognitive remediation for negative symptoms of schizophrenia: A network meta-analysis. Clin Psychol Rev. 2017;52:43–51. [DOI] [PubMed] [Google Scholar]
- 70. Otis JM, Namboodiri VM, Matan AM et al. Prefrontal cortex output circuits guide reward seeking through divergent cue encoding. Nature. 2017;543:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Carus-Cadavieco M, Gorbati M, Ye L et al. Gamma oscillations organize top-down signalling to hypothalamus and enable food seeking. Nature. 2017;542:232–236. [DOI] [PubMed] [Google Scholar]
- 72. Ferenczi EA, Zalocusky KA, Liston C et al. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science 2016;351:aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]