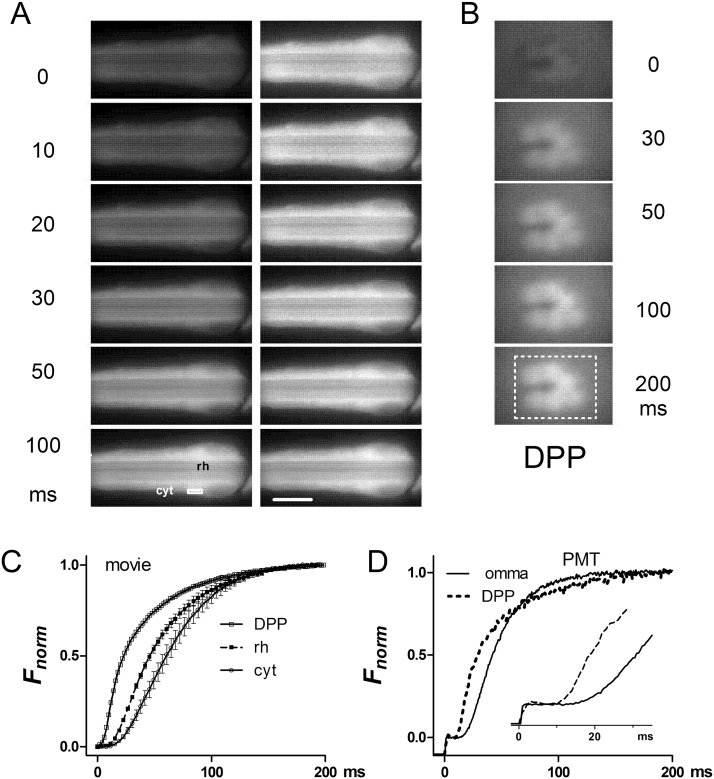
Fig. 2.
Live imaging of GCaMP6f.
(A) GCaMP6f fluorescence in a dissociated ommatidium (distal end) from ninaE-GCaMP6f fly: 6 frames from 250 Hz movie (4 ms exposures; see Movie 1) at t = 0 to 100 ms after turning on blue excitation. Images on left are raw images with brightness and contrast adjusted with respect to the same (brightest) frame; images on right are the same but with brightness and contrast individually auto-adjusted. Scale bar 10 μm (×40 oil immersion objective). (B) Frames from a similar movie of GCaMP6f in rhabdomeres imaged in the deep pseudopupil (DPP) of an intact living ninaE-GCaMP6f fly (x 20 air objective). (C) Time-courses from movies (as in A & B). In dissociated ommatidia, regions of interest from rhabdomeres (rh) and cytosol (cyt) towards edge of the ommatidium (white box in A) were selected. For DPP a rectangle encompassing all 6 rhabdomeres was selected. Mean ± S.E.M. n = 5-6 ommatidia. Traces normalised to facilitate comparison of time course: maximum ΔF/F0 values were in range 11–18 (see Fig. 3). (D) Normalised raw photomultiplier tube traces (PMT) sampled at 1 kHz, filtered at 0.5 kHz from ninaE-GCaMP6f flies. Representative single traces in response to supersaturating blue excitation are shown recorded from a dissociated ommatidium in normal bath (omma) and in vivo from the deep pseudopupil (DPP). Rising phases of the same traces shown in inset.