Figure 2. Structure activity relationships of cisplatin analogs.
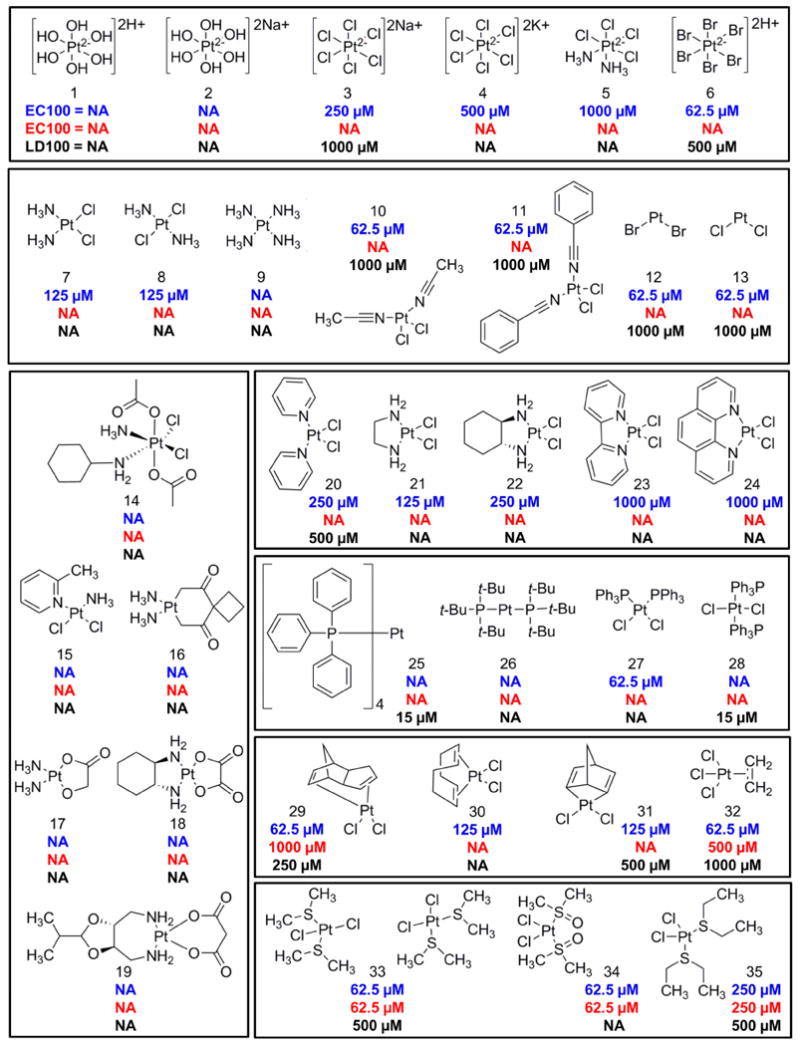
A panel of 35 cisplatin analogs was grouped into the following classes: platinum (IV) (1-6), square planar (7-13), FDA approved (14-19), pyridine (20-24), triphenylphosphine (25-28), alkene (29-32), and sulfur-containing complexes (33-35). A ten point dose curve ranging from 1-1000 μM was tested. The doses that rescued 100% of zebrafish (EC100) from a challenge with 100 μM KCN were determined. The EC100 was determined in both DMSO and PBS solvents (blue and red, respectively). In a separate assay, the doses that caused 100% lethality (LD100) in the absence of KCN were determined for complexes dissolved in DMSO (black). NA indicates instances in which the complex did not induce any toxicity or did not rescue cyanide lethality at any of the doses tested.
