Successful clinical trials of antiretroviral pre-exposure prophylaxis (PrEP) to prevent HIV infection have been reported in heterosexual men and women, men who have sex with men (MSM), and injection drug users1. A daily oral drug regimen containing nucleoside reverse transcriptase inhibitors (NRTIs) tenofovir disoproxil fumarate (TDF) alone or in combination with emtricitabine (FTC) have been effective when participant adherence is high1. Analysis of PrEP dosing patterns estimate taking at least four TDF doses per week provides 96% protection from HIV infection and at least two doses per week provides 76% protection in MSM and transgender women2, though some estimate more frequent dosing in heterosexual women may be needed3. TDF and FTC require phosphorylation in HIV target cells to tenofovir-diphosphate (TFV-DP) and emtricitabine-triphosphate (FTC-TP) to provide PrEP protection as competitive analogs of naturally occurring deoxyadenosine triphosphate (dATP) and deoxycytidine triphosphate (dCTP), respectively. However, it is unclear if physiological conditions that increase dNTP concentrations can affect pharmacokinetics (PK) and pharmacodynamics (PD) of NRTIs. This may be particularly relevant when cellular deoxynucleoside triphosphate (dNTP) pools in HIV target cells increase in response to immune activation. Decreased ratios of phosphorylated NRTIs to their respective dNTPs have been associated with cell activation in vitro and in nonhuman primate studies4,5, yet this observation remains unexplored in PK and PD studies that measured intracellular drug6-8. The study presented here compared dATP and dCTP concentrations to TFV-DP and FTC-TP in peripheral blood mononuclear cell (PBMCs) of persons using daily oral Truvada™ during a successful HIV PrEP study. We further examined if variations among intracellular drug:dNTP ratios were related to lymphocyte activation.
PBMCs were isolated from 12 persons before (T0) and 2, 4, and 8 hours following an observed single daily dose of Truvada™ during their 3-month study visit for the TDF2 PrEP Trial, so samples should be at pseudo-steady-state9. Prescribed dosing at bedtime resulted in assumed T0 sampling approximately 18-24 hours following the previous dose. Intracellular active drug metabolites TFV-DP, FTC-TP, and their natural substrates, dATP and dCTP, respectively, were measured in methanol extracts of PBMCs by high performance liquid chromatography-tandem mass spectrometry as previously described5,10. A wide range of presumed steady-state intracellular concentrations was observed for TFV-DP (median = 64 fmol/106 PBMC; range: 20-387 fmol/106 PBMC) and FTC-TP (median = 6451 fmol/106 PBMC; range: 1361-30310 fmol/106 PBMC), yet concentrations of TFV-DP and FTC-TP were highly correlated (Spearman Rank Order, ρ = 0.860, p < 0.001).
To estimate adherence to PrEP dosing in our substudy, we compared our intracellular TFV-DP and FTC-TP concentrations to those reported in the HPTN 066 study of observed Truvada™ dosing7. This comparison estimated that all of our substudy participants were taking four or more Truvada™ doses per week (TFV-DP >19 fmol/106 PBMC, FTC-TP >600 fmol/106 PBMC), while 10 had TFV-DP concentrations (>36 fmol/106 PBMC) and 11 had FTC-TP concentrations (>2200 fmol/106 PBMC) consistent with daily dosing. Within the 8 hour sampling window after the observed Truvada™ dose, all 12 participants' TFV-DP and FTC-TP concentrations were at or above those reported for observed daily dosing in HPTN 066.
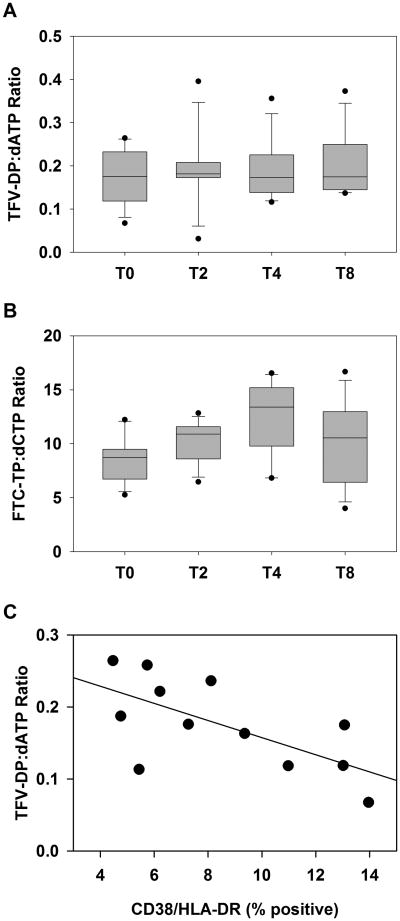
There were also wide ranges of concentrations for both dATP (median = 430 fmol/106 PBMC; range: 89-2375 fmol/106 PBMC) and dCTP (median = 776 fmol/106 PBMC; range: 226-1635 fmol/106 PBMC) in the PBMC samples. However, these nucleotide concentrations were highly correlated with their respective intracellular drug analog concentrations in the pre-dose sample (T0) (TFV-DP and dATP, ρ = 0.881, p < 0.001; FTC-TP and dCTP, ρ = 0.909, p < 0.001). The ratio of phosphorylated drug to dNTP in PBMCs was lower for TFV-DP:dATP (median, 0.18:1) than for FTC-TP:dCTP (median, 8.73:1). The TFV-DP:dATP ratio remained mostly unchanged throughout the 8 hour post-dose sampling period (Figure 1A) while the FTC-TP:dCTP ratio increased from pre-dose (T0) to 4 hours (p < 0.001) before declining at 8 hours post dose (Figure 1B). Plasma TFV and FTC concentrations at these time points peaked at 2-4 hours post-dose as expected and were not correlated with TFV-DP, FTC-TP, or dNTP levels (data not shown).
Figure 1.
Intracellular tenofovir-diphosphate and emtricitabine-triphosphate concentrations correlate with corresponding natural substrate concentrations. Intracellular ratios concentrations of NRTI and dNTP were calculated in PBMCs pre-dose (T0) and 2, 4 or 8 hours post-dose for TFV-DP:dATP (A) and FTC-TP:dCTP (B). The ratio of TFV-DP to dATP concentrations were calculated for T0 samples and cellular activation was determined by the percentage of CD4+ lymphocytes stained CD38+/HLA-DR+ by flow cytometry (C).
To assess whether cell activation was associated with intracellular drug concentrations and NRTI:dNTP ratios, we measured the percentage of CD4+ lymphocytes expressing CD38 and HLA-DR in cryopreserved T0 PBMC samples using a Multitest antibody cocktail (CD4/CD38/CD3/HLA-DR, BD Biosciences, San Jose, CA) and FACSCalibur flow cytometer (BD Biosciences). The CD38+/HLA-DR+ population ranged from 4 to14% of CD4+ lymphocytes and inversely correlated with TFV-DP:dATP ratios of PBMCs (ρ= -0.608, p = 0.033; Figure 1C). However, neither FTC-TP:dCTP ratios, individual intracellular drug concentrations, nor dATP or dCTP concentrations were correlated with CD38+/HLA-DR+ expression (all p > 0.05, data not shown).
The results of our substudy provide new intracellular PK data for heterosexual adults enrolled in a successful PrEP trial of daily oral dosing of Truvada™. The intracellular TFV-DP and FTC-TP concentrations we observed were similar in range to steady-state concentrations reported for HIV-negative participants in a pharmacokinetic study (TFV-DP mean, 103 fmol/106 cells; FTC-TP mean, 6 pmol/106 cells)11 which are associated with dosing regimens providing protection among MSM participants in a PrEP study that demonstrated a reduced risk for acquiring HIV6. However, analyses of larger data sets are needed to adequately determine if the TFV-DP or FTC-TP concentrations and NRTI:dNTP ratios we report reflect predictive PrEP-effective concentrations for at-risk heterosexual persons.
The pseudo-steady-state NRTI:dNTP ratios reported here remained consistent throughout a broad range of intracellular drug concentrations in PBMCs, suggesting NRTI:dNTP ratios may not vary widely among adherent PrEP study participants. Yet, within our limited data set, lower TFV-DP:dATP ratios associated with increased PBMC activation suggesting immune activation may influence intracellular ratios in vivo similar to that reported for PBMC activation in vitro4. Immune activation may increase dATP or decrease TFV-DP thereby reducing the TFV-DP:dATP ratio, but no consistent trend was observed for either possibility in the study reported here. FTC-TP:dCTP ratios were not correlated with cell activation in this study which may be related to metabolic factors that may include the markedly shorter intracellular half-life reported for FTC-TP (17 hours) compared to TFV-DP (5.6 days)12. Previous studies indicated dNTP pools in PBMCs decline following initiation of TDF/FTC therapy in both HIV-negative and HIV-positive individuals13, and TDF/FTC therapy can reduce immune activation among HIV-negative individuals14. These studies and the results presented here suggest a complex interplay between antiretroviral therapy and cell activation establishes drug:dNTP ratios in PBMCs.
Limitations to our study are that drug:dNTP measurements were performed using the entire pool of PBMCs, while activation was examined only for the subpopulation of CD4+ PBMCs and our analysis did not include genital or rectal mucosal tissues where sexually transmitted virus exposure occurs. While the role of mucosal tissue drug concentrations and drug:dNTP ratios in protecting against HIV has yet to be defined, a wide range of TFV-DP:dATP ratios in human mucosal tissues8 and reduced TFV-DP:dATP ratios in rectal lymphocytes in a non-human primate model5 have been reported. Mucosal NRTI:dNTP ratios are likely more variable than those observed in PBMCs, yet clinical trials continue to report high levels of protection among adherent participants indicating that within these widely fluctuating ratios, a high degree of efficacy is maintained9. Nevertheless, examination of NRTI:dNTP ratios at mucosal sites of HIV exposure and their role in PrEP efficacy is warranted, especially under physiological conditions that increase immune activation such as sexually transmitted infections.
Acknowledgments
The authors would like to thank the TDF2 study participants for their contribution of time, energy and commitment to this effort. Financial support for this study was provided by the United States Centers for Disease Control and Prevention.
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the views of the United States Centers for Disease Control and Prevention or the Department of Health and Human Services.
Financial support for this study was provided by the United States Centers for Disease Control and Prevention.
References
- 1.Baeten JM, Haberer JE, Liu AY, Sista N. Preexposure prophylaxis for HIV prevention: where have we been and where are we going? J Acquir Immune Defic Syndr. 2013;63(2):S122–129. doi: 10.1097/QAI.0b013e3182986f69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–829. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cottrell ML, Prince HM, Allmon A, et al. Cervicovaginal and Rectal Fluid as a Surrogate Marker of Antiretroviral Tissue Concentration: Implications for Clinical Trial Design. J Acquir Immune Defic Syndr. 2016 doi: 10.1097/QAI.0000000000000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins BL, Wilcox CK, Fridland A, Rodman JH. Metabolism of tenofovir and didanosine in quiescent or stimulated human peripheral blood mononuclear cells. Pharmacotherapy. 2003;23(6):695–701. doi: 10.1592/phco.23.6.695.32189. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Lerma JG, Aung W, Cong ME, et al. Natural substrate concentrations can modulate the prophylactic efficacy of nucleotide HIV reverse transcriptase inhibitors. J Virol. 2011;85(13):6610–6617. doi: 10.1128/JVI.00311-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendrix CW, Andrade A, Bumpus NN, et al. Dose frequency ranging pharmacokinetic study of tenofovir-emtricitabine after directly observed dosing in healthy volunteers to establish adherence benchmarks (HPTN 066) AIDS Res Hum Retroviruses. 2015 doi: 10.1089/aid.2015.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cottrell ML, Yang KH, Prince HM, et al. A Translational Pharmacology Approach to Predicting Outcomes of Preexposure Prophylaxis Against HIV in Men and Women Using Tenofovir Disoproxil Fumarate With or Without Emtricitabine. J Infect Dis. 2016 doi: 10.1093/infdis/jiw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 10.Kuklenyik Z, Martin A, Pau CP, et al. On-line coupling of anion exchange and ion-pair chromatography for measurement of intracellular triphosphate metabolites of reverse transcriptase inhibitors. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2009;877(29):3659–3666. doi: 10.1016/j.jchromb.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Seifert SM, Glidden DV, Meditz AL, et al. Dose response for starting and stopping HIV preexposure prophylaxis for men who have sex with men. Clin Infect Dis. 2015;60(5):804–810. doi: 10.1093/cid/ciu916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seifert SM, Chen X, Meditz AL, et al. Intracellular Tenofovir and Emtricitabine Anabolites in Genital, Rectal, and Blood Compartments from First Dose to Steady State. AIDS Res Hum Retroviruses. 2016;32(10-11):981–991. doi: 10.1089/aid.2016.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Castillo-Mancilla JR, Seifert SM, et al. Analysis of the Endogenous Deoxynucleoside Triphosphate Pool in HIV-Positive and -Negative Individuals Receiving Tenofovir-Emtricitabine. Antimicrob Agents Chemother. 2016;60(9):5387–5392. doi: 10.1128/AAC.01019-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castillo-Mancilla JR, Meditz A, Wilson C, et al. Reduced immune activation during tenofovir-emtricitabine therapy in HIV-negative individuals. J Acquir Immune Defic Syndr. 2015;68(5):495–501. doi: 10.1097/QAI.0000000000000529. [DOI] [PMC free article] [PubMed] [Google Scholar]