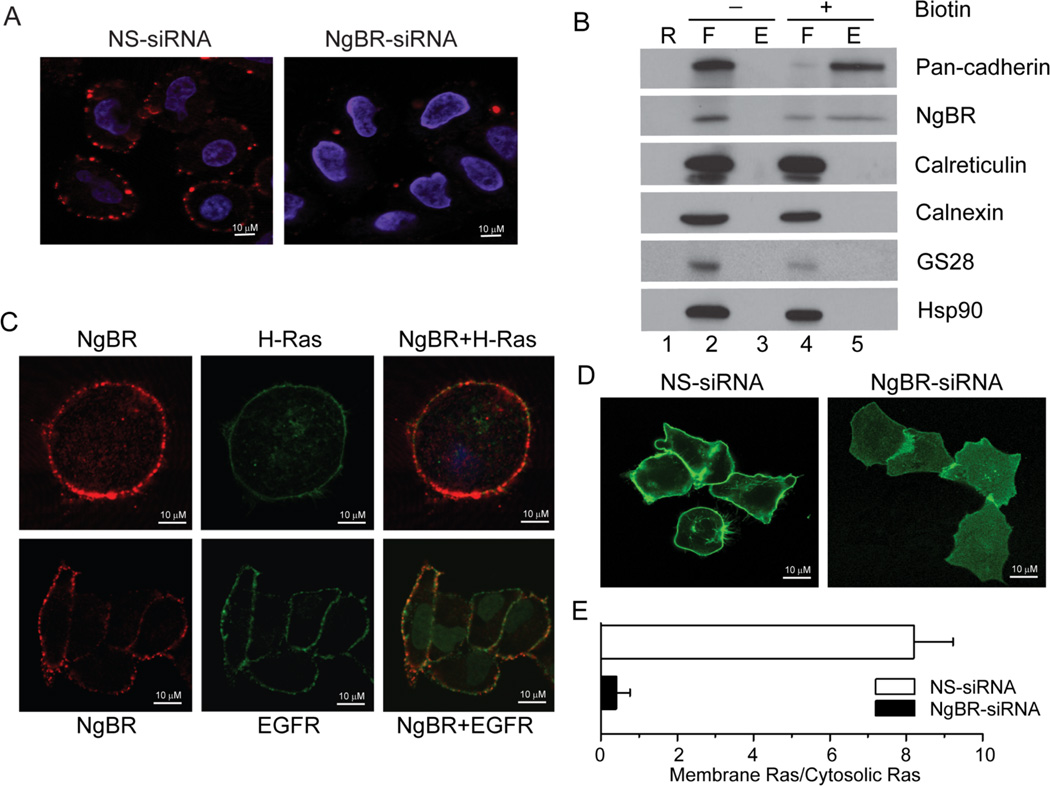
Figure 1. NgBR is essential for H-Ras plasma membrane localization.
(A) NgBR is localized in the plasma membrane of non-permeabilized HeLa cells. Immunofluorescence staining was performed using NgBR rabbit polyclonal antibody specially recognizing the ecto-domain of NgBR. (B) NgBR is detected in the fraction of biotinylated cell surface proteins. HeLa cell surface proteins were biotinylated under non-permeabilized conditions and isolated using streptavidin agarose resin from the Pierce Cell Surface Protein Isolation Kit. Proteins were detected by Western blot analysis. Pan-Cadherin, Calreticulin and GS28 are plasma membrane, ER membrane and Golgi membrane markers, respectively. The plus symbol (+) denotes results for cells treated with the Sulfo-NHS-SS-Biotin reagent; the minus symbol (−) denotes results for cells that were not treated with the biotin reagent but were otherwise carried through the kit procedure. The lane designated “R” shows control samples that were isolated in the absence of avidin-agarose resin, the lanes designated “F” show proteins that flowed through the columns because they did not bind the avidin-agarose resin, and the lanes designated “E” show proteins that were eluted from the columns after binding to the avidin-agarose resin. As expected, surface proteins bound to the avidin-agarose resin if the cells were labeled with biotin (“E”, lane 5), but surface proteins flowed through the columns if the cells were not labeled with biotin (“F”, lane 2). (C) NgBR is co-localized with EGFP-H-Ras in EGFP-H-Ras transfected HeLa cell (upper panel) and NgBR is partially co-localized with EGFR in HeLa cell plasma membrane (bottom panel). (D) NgBR knockdown impaired the plasma membrane localization of EGFP-H-Ras in HeLa cells. (E) Quantification results were presented as the ratio of cells showing EGFP-H-Ras plasma membrane location to cells showing EGFP-H-Ras cytoplasma location. Data are represented as mean ± SEM (* P< 0.05, n=3).