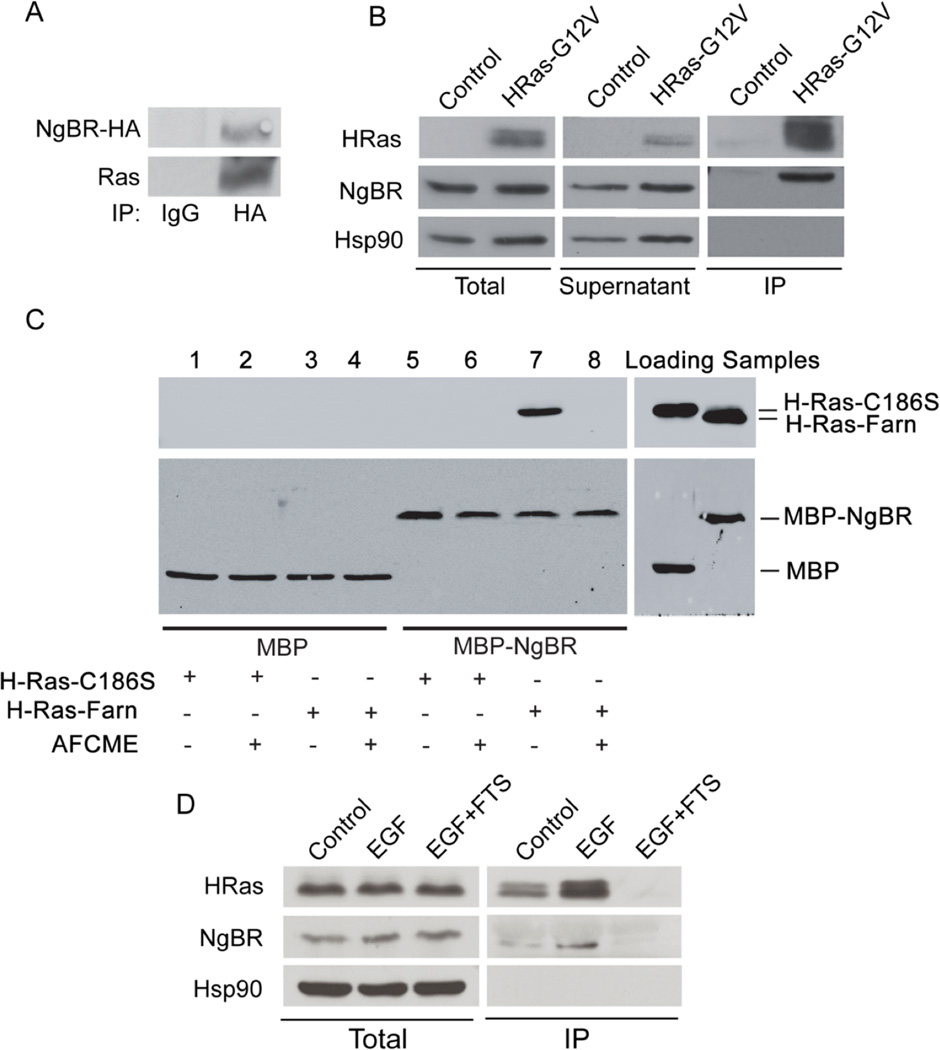
Figure 2. Prenylation of H-Ras is essential for NgBR-Ras interaction.
(A) Endogenous Ras was detected in the complex of NgBR-HA immunoprecipitated from 293T cells transfected with NgBR-HA plasmid DNA. (B) Endogenous NgBR interacts with the constitutively activated H-Ras. Plasmid DNA of H-Ras-G12V mutant was transfected to HeLa cells. The complex of activated Ras (GTP-loaded Ras) was precipitated from the quiescent cells using GST-RBD beads. Protein levels were detected by Western blotting. Both Ras and endogenous NgBR were detected in the complex precipitated by the Raf-pull-down method. (C) NgBR interacts with Ras directly in a cell-free system. AFCME: a farnesyl analog, N-Acetyl-S-farnesyl-L-cysteine-methyl ester. Protein levels were detected by Western blotting. Purified MBP (Maltose binding protein)-tagged NgBR (MBP-NgBR) was incubated with either unfarnesylated Ras (H-Ras-C186S, containing a mutation at the cysteine of CAAX box) or farnesylated Ras (H-Ras-Farn). The complex was immunoprecipitated using anti-MBP magnetic beads (New England BioLabs). (D) Blocking prenyl-dependent interactions of H-Ras diminishes its interactions with NgBR. HeLa cells were stimulated with 100 ng/ml EGF for 5 minutes. The complex of the GTP-loaded H-Ras was pulled down using GST-RBD beads. FTS pretreatment (20µM for overnight) abolished the interaction of GTP-loaded H-Ras and NgBR. FTS: S-trans, trans-farnesylthiosalicylic acid. Data are validated in 3 independent experiments.