Figure 6.
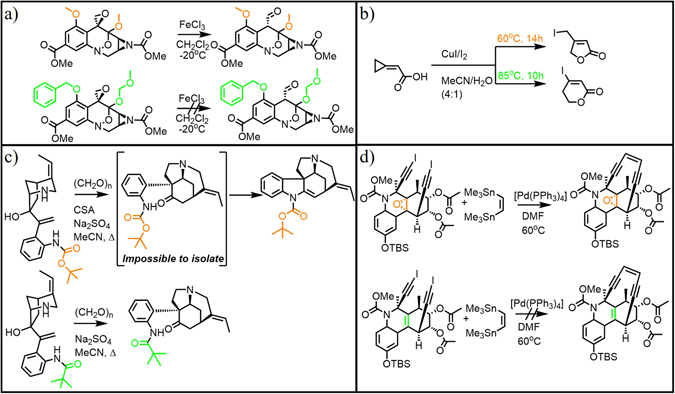
A challenge for Machine Learning: Minor structural changes in starting materials can dramatically influence the reaction outcomes. (a) Replacement of two O-protecting groups (orange OMe to green OBn and OMOM) in the intermediate in Danishefsky’s synthesis of (+/−)-FR-900482 changes the lability of ether groups and prohibits rearrangement of an epoxide to an aldehyde37. (b) Minute changes in temperature alter reaction mechanism and result in different products38. (c) Small changes in electron density modify reactivity of N-pivaloyl and N-Boc protected anilines. The upper substrate reacts into an intermediate that is impossible to isolate and thus leads to a product that is markedly different than the one obtained from the lower substrate differing in only one atom (oxygen)39. (d) Presence of the epoxide ring in the tricyclic moiety allows for close proximity of the terminal iodides enabling double Pd-mediated coupling. In contrast, when the epoxide is replaced by a double bond, the iodides are further apart and no cyclization is observed40.
