Abstract
This study aimed to examine clinical features, sleep, abnormal sleep-wake transition and non-sleep disturbances as well as lab tests in Chinese fatal familial insomnia (FFI) subjects. Patients with confirmed clinical and laboratory diagnosis of FFI have been retrospectively reviewed. The clinical features and the results of the complementary tests, including polysomnography (PSG), brain imaging and genetic analysis, were used. Two male and three female patients were recruited in this study. Three of the five patients had more comprehensive family medical records. The most typical clinical manifestations in all 5 patients were sleep disturbances, including insomnia, laryngeal stridor, sleep breath disturbance, and sleep-related involuntary movements. PSG of all these five cases showed reduction in total sleep time, sleep fragmentation, abnormal short non-rapid eye movement - rapid eye movement (REM) cycling, REM sleep reduction or loss, and REM sleep instruction in wakefulness. Patient 2's emission tomography scan demonstrated a reduction in glucose uptake in the left thalamus and bilateral inferior parietal lobe. In summary, Chinese FFI patients are typically characterized by organic sleep related symptoms, rapidly progressive dementia and sympathetic symptoms. We propose that structural damages in the thalamus and cortex are mostly responsible for clinical manifestations of FFI.
Introduction
Fatal familial insomnia (FFI) is a rare prion disease first described by Lugaresi et al., in 19861. The prevalence of FFI is one case per a million population per year, with only about 57 cases in 27 kindreds have been reported worldwide2.
FFI is an autosomal dominant disease that harbors a missense GAC to AAC mutation at codon 178 of the PRNP prion protein gene located on chromosome 20, along with the presence of the methionine polymorphism at position 129 of the mutant allele3, 4. Pathologically, FFI is characterized by predominant thalamic degeneration especially in the medio-dorsal and anterio-ventral nuclei5, 6. Phenotypic variability is a perplexing feature of FFI. FFI is mainly characterized by prominent sleep impairment combined with neuropsychiatric disorders, dysautonomia and motor dysfunction7. Polysomnography (PSG) recordings document the biophysiological changes that occur during sleep. In FFI, PSG has disclosed a severely reduced total sleep time, reduced rapid eye movement (REM) sleep and abnormal stage shifts. In some cases, FFI was characterized by the lack of REM-associated muscle atonia and the presence of jerky activity of limb muscles and irregular breathing8.
Irregular breathing, hypnic jerks, propriospinal myoclonus at the wake-sleep transition, and quasi-purposeful limb gestures are considered to be characteristic features of FFI6, 7. Further, husky voice was reported in 22% of FFI patients in Germany9. Nevertheless, the mechanism and diagnostic value of sleep related respiratory disturbance and movement disorder in FFI have not been studied thoroughly yet. Here, we have analyzed the clinical manifestations and biological changes, especially the sleep related symptoms, PSG and brain imaging results of five Chinese patients diagnosed with FFI. This study aims to understand the pathogenesis of sleep related disorders in FFI.
Results
Clinical characteristics and neurological features
A total of five FFI patients of Chinese Han decent (2 males and 3 females) were enrolled in the current study. The median age at FFI onset was 46.4 years (from 19 to 62 years) and the duration of the course of FFI ranged from 8 to 18 months. The most prominent clinical manifestations in all five patients was sleep disturbances that included insomnia, laryngeal stridor, sleep breath disturbance, oneiric or stuporous episodes with hallucinations and confusion, and sleep-related involuntary movements (such as hypnic jerks, restless sleep with frequent changes in body position and twitchy non-purposeful movement of limbs). Furthermore, we have observed the development of a rapidly progressive dementia (RPD) along with psychiatric symptoms in all patients. MMSE (mini-mental state examination) scores of case 1 and case 2 were only 4 (Normal reference ≥ 20), CDR (clinical dementia rating) of case 5 was 2. Other patients had severe dementia and they could not complete cognitive scales. Progressive sympathetic symptoms i.e. hypertension, sweating, tachycardia, and irregular breathing were also presented by the patients enrolled in this study. Ataxia, pyramidal sign, extrapyramidal sign and dysarthria were also observed. The main clinical features and neurological features of these five cases are summarized in Table 1.
Table 1.
Clinical characteristics of the five FFI patients.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Gender | M | F | F | F | M |
| Age at onset (years) | 62 | 60 | 19 | 36 | 55 |
| Onset to diagnosis duration (months) | 3 | 5 | 3 | 8 | 11 |
| Onset to death duration (months) | 12 | 11 | 8 | 10 | 18 |
| Cluster A—sleep-related symptoms | |||||
| Insomnia | + | + | + | + | + |
| Sleep-related involuntary movements | + | + | + | + | + |
| Sleep-related dyspnea | + | + | + | + | + |
| Laryngeal stridor | + | + | + | + | + |
| Cluster B—neuropsychiatric symptoms | |||||
| RPD | + | + | + | + | + |
| Psychiatric symptoms | + | + | − | + | + |
| Ataxia | − | + | + | + | + |
| Pyramidal sign | − | − | + | + | − |
| Parkinsonism | − | − | − | − | + |
| Cluster C–progressive sympathetic symptoms | |||||
| Autonomic symptoms | |||||
| Hypertension | + | + | − | + | − |
| Sweating | + | + | − | + | + |
| Tachycardia | + | − | + | + | − |
| Irregular breathing | − | − | + | − | − |
RPD: rapidly progressive dementia. +: symptom/sign observed, −: symptom/sign not observed. Psychiatric syndromes included hallucination, personality change, depression, anxiety, aggressiveness, disinhibition, listlessness etc. Autonomic signs include hypertension, sweating, tachycardia, irregular breathing etc. Sleep-related involuntary movements included frequent changes in the body position and twitchy non-purposeful movements of limbs during sleep.
Familial medical history and genetic analysis
Patients 1 and 2 were siblings, and interviews with family members have noted that their mother and an elder brother had similar FFI symptoms. Furthermore, the mother of Patient 5 died at the age of 70 years old due to rapid progressive dementia (RPD). The other two patients (Patients 3 and 4) had no known family history.
Gene sequencing of DNA extracted from the peripheral blood leukocytes revealed that all five patients had a D178N mutation with methionine/methionine homozygosity at the polymorphic 129 codon of the PRNP gene. Seven family members of Patients 1 and 2 were screened for PRNP gene mutation. Other family members were not screened due to lack of consent or prior death. Results of the screened family members demonstrated that the healthy control subjects number II-6, III-6, III-11, III-13, and III-14 were free of the PRNP D178N mutation and subjects number III-10 and III-15 were clinically healthy carriers of the PRNP D178N mutation (Fig. 1). Patient 3’s mother was also screened for PRNP gene mutation, and she was also a carrier of the D178N mutation without displaying the clinical FFI symptoms (data not shown).
Figure 1.
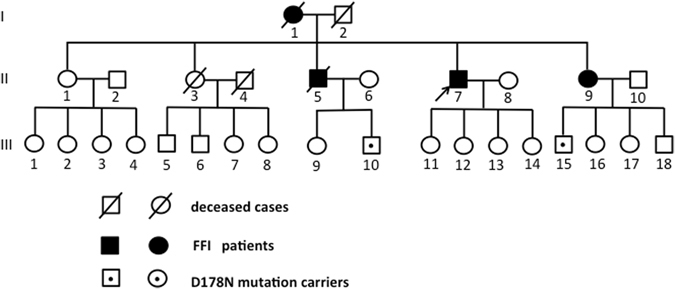
The pedigree of case 1(II-7) and case 2(II-9). II-7 is the proband of this pedigree (arrow). II-7 and II-9 are respectively Patient 1 and Patient 2 who carried PRNP D178N mutation. Additionally, seven family members were screened for PRNP gene mutation: II-6, III-6, III-11, III-13, and III-14 were healthy participant free of PRNP D178N mutation; III-10 and III-15 were clinically healthy carriers of the PRNP D178N mutation. Other family members were not screened due to lack of consent or prior death.
The expression of 14-3-3 protein, EEG analysis, and brain imaging
ELISA results confirmed that all patients were negative for the CSF 14-3-3 protein, consistent with previous reports indicating that 14-3-3 protein is usually normal or nonspecifically changed in FFI10. EEG showed diffuse slow waves without periodic spike discharges in the five patients, and the brain MRI did not reveal any abnormalities (data not shown). SPECT performed on Patient 1 indicated a decline in blood perfusion of the bilateral thalamus, basal ganglia, and the medial temporal lobe (Fig. 2). Furthermore, MRS analysis for Patient 1 showed a declined level of N-aceytl aspartate (NAA) in the left thalamus (data not shown). Patient 2 underwent a PET scan, which demonstrated decreased glucose uptake in the left thalamus and bilateral inferior parietal lobe (Fig. 3).
Figure 2.
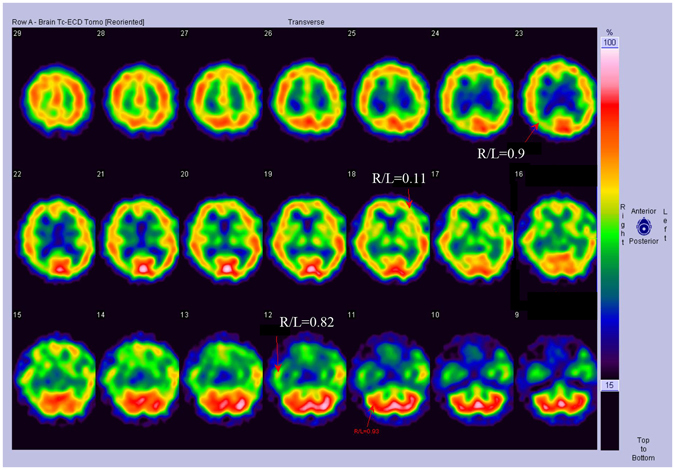
SPECT analysis of FFI patients. Patient 1 SPECT image showing reduced blood flow perfusion in bilateral temporal lobes, bilateral basal ganglia, and bilateral thalamus.
Figure 3.
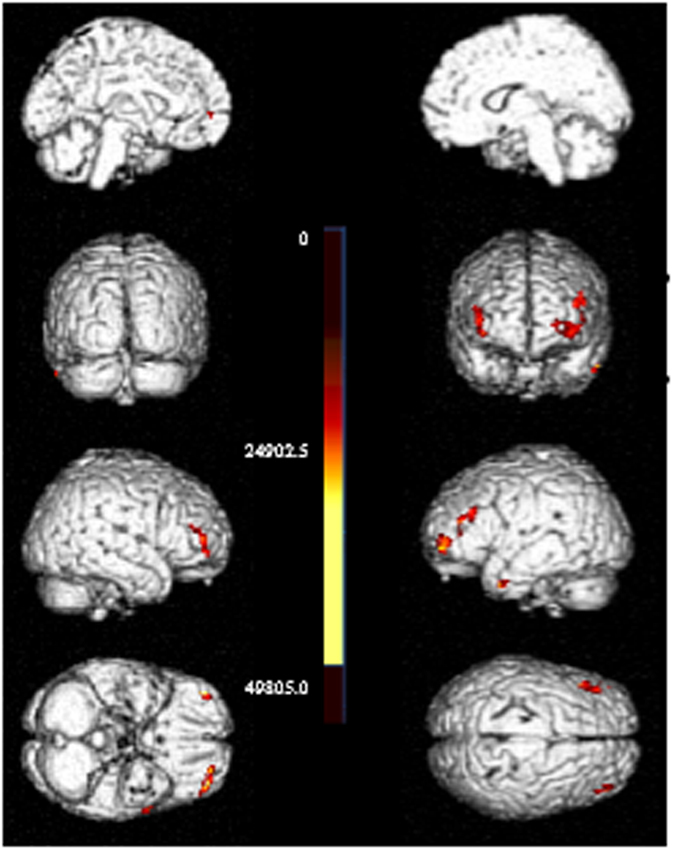
PET analysis of FFI patients. PET image for Patient 2 indicating reduced glucose metabolism in the bilateral frontal and parietal lobes and left thalamus.
PSG analysis shows signs of sleep-related respiratory disturbance and leg-movement
All five patients enrolled in this study underwent PSG. The results demonstrated an early reduction in the sleep spindles and K complexes, a drastic reduction in the total sleep time by about 50% (average total sleep amounts = 212.3 ± 26.6 minutes), and decreased sleep efficiency. In addition, disruptions of the normal cyclic sleep organization, especially the loss or decreased ratio of REM phase and prolonged sleep latency of REM were also detected. In Patient 3, REM sleep occurred directly from wakefulness. The PSG indices for sleep efficiency and sleep organization are listed in Table 2. Moreover, PSG identified sleep apnea syndrome in all FFI patients, characterized by obstructive apnea/hypopnea and laryngeal stridor in the NREM stage. We observed involuntary movements in these five patients during sleep, mostly during the NREM stage 2. We did not detect PLMS. The PSG indices for sleep-related respiratory disturbance and leg-movement are listed in Table 3.
Table 2.
Sleep efficiency and sleep organization indices for the five FFI patients.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Ref. | |
|---|---|---|---|---|---|---|
| Time in bed (min) | 509.0 | 513.0 | 593.5 | 549.5 | 614.0 | 419–464 |
| Total sleep time (min) | 167.0 | 269.5 | 145.5 | 277.5 | 202.0 | 402–449 |
| Sleep efficiency (%) | 32.8 | 52.5 | 24.5 | 50.5 | 32.9 | 85–98 |
| NREM Stage 1 (%) | 0.6 | 5.2 | 3.8 | 4.1 | 5.0 | 2–5 |
| NREM Stage 2 (%) | 32.9 | 24.1 | 68.0 | 31.4 | 59.2 | 45–55 |
| NREM Stage 3 (%) | 66.5 | 70.7 | 16.2 | 61.3 | 25.5 | 20–25 |
| REM (%) | 0 | 0 | 12.0 | 3.2 | 10.4 | 20–25 |
| Latency to REM stage (min) | — | — | 339.0 | 208.5 | 117.5 | 90–110 |
Ref. stands for reference value range; REM: rapid eye movement; NREM: Non- rapid eye movement.
Table 3.
Sleep-related respiratory disturbance and leg-movement indices of the five FFI patients.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Ref. | |
|---|---|---|---|---|---|---|
| BMI | 23 | 20.8 | 19.2 | 19.5 | 20.5 | 18–24 |
| Baseline oxygen saturation | 95 | 97 | 97 | 97 | 94 | |
| Minimum oxygen saturation | 83 | 89 | 86 | 91 | 76 | |
| AHI | 10.8 | 7.8 | 41.6 | 4.3 | 18.7 | <5 |
| Obstructive apnea | + | + | + | + | + | − |
| Laryngeal stridor | + | + | + | + | + | − |
| NREM limb movements | 425 | 951 | 225 | 557 | 229 | − |
| REM limb movements | 0 | 0 | 0 | 2 | 4 | − |
| PLMS index | 0 | 0 | 0 | 0 | 0 | <5 |
AHI: apnea/hypopnea index; PLMS: periodic leg movement in sleep; +: symptom/sign observed; −: symptom/sign not observed.
Hypnogram analysis
Finally, the hypnogram analysis for the five FFI patients has revealed a decreased sleep efficiency and disruption of the normal cyclic sleep organization. In contrast, the healthy control participants successfully completed several sleep cycles (Fig. 4).
Figure 4.
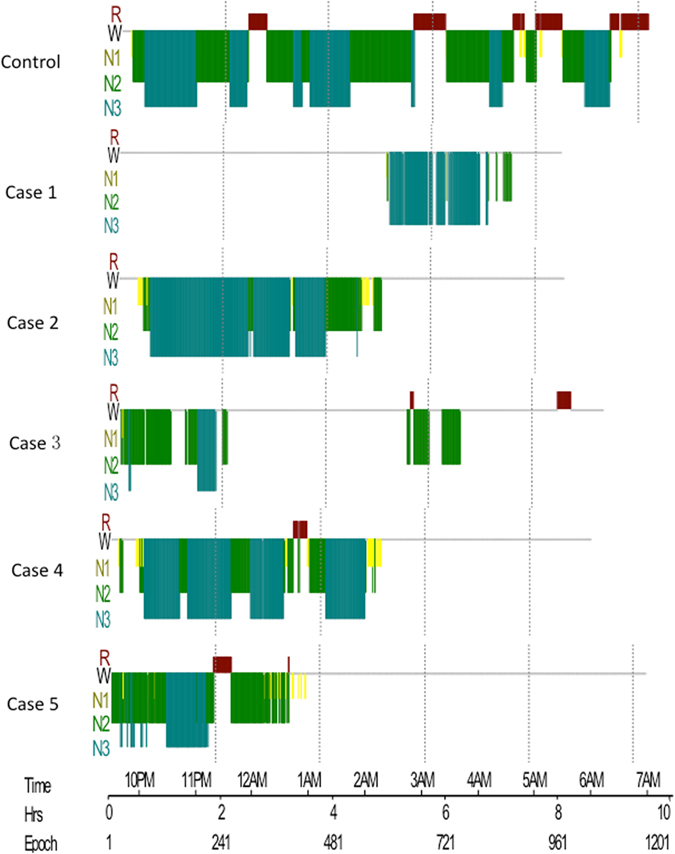
Hypnogram of a healthy control and the five FFI patients. The 5 FFI patients presented with decreased sleep efficiency and disruption of the normal cyclic sleep organization (horizontal axis in sleep hours). W: wake, R: REM, N1-3: NREM sleep stages.
Discussion
FFI is a rare fatal genetic disease that affects both genders equally. The mean age at disease onset is approximately 50 years (range, 21–62 years), and the FFI duration varied from 7 to 72 months7, 11. Increased insomnia and sleep-wake cycle disruption are the clinical hallmarks of FFI. In this study, we reported the clinical aspects as well as the analysis of sleep cycle and sleep-related disturbances for five Chinese Han patients. We have compared symptoms of Chinese FFI patients with FFI patients reported from other countries, especially those from Italy and Germany, and no major differences were found. Patient 3 had an early onset of FFI at the age of 19 years. To the best of our knowledge, this is the youngest FFI patient ever reported. Genetic analysis of the PRNP gene confirmed that Patient 3’s mother was a healthy carrier of the D178N mutation, suggesting that PRNP gene mutation may have an autosomal dominant trait with incomplete penetrance.
The alterations in the sleep cycle in the five FFI cases were investigated by PSG recording. All patients showed a declined sleep efficiency and disruption of the cyclic sleep organization. The recording revealed abortion of the REM sleep or reduction of REM and increased latency to reach the REM stage. Furthermore, PSG results revealed an early reduction in the sleep spindles and K complexes in our patients, and these results came in accordance with previous studies1–3. Sleep spindles and K-complexes indicate the transition from light to deep NREM sleep. It has been confirmed that sleep spindles are produced by the thalamus12. Therefore, the loss of the sleep spindles and K-complexes suggests a decline in thalamic function8.
In this study, we discovered characteristic sleep features that were rarely reported in previous FFI cases, i.e., sleep apnea syndrome, laryngeal stridor, and sleep-related involuntary movements. In good agreement with these clinical symptoms, PSG recorded obstructive apnea/hypopnea, laryngeal stridor, and NREM stage 2 involuntary movement in the five patients enrolled in this study. Therefore, it is plausible to speculate that active laryngeal narrowing during inspiration led to the development of obstructive apnea/hypopnea and laryngeal stridor in patients with FFI. The paralysis of the posterior cricoarytenoid muscle was reported to cause passive laryngeal narrowing producing the inspiratory stridor13. In the five cases enrolled in this study, we did not observe PLMS events; however, we noticed involuntary limb movements, especially in NREM stage 2. This result came in accordance with previous studies that reported hypnic jerks, quasi-purposeful limb gestures, and propriospinal myoclonus atypical to the NREM stage2, 6, 7. It was proposed that the abnormal involuntary movements during sleep were attributed to the thalamus14, 15.
The histopathological hallmark of FFI is severe neuronal loss and deposition of the pathogenic prion protein (PrPSc)16. Previous postmortem neuropathological studies revealed severe atrophy of the thalamus, particularly in the anteroventral and dorsomedial thalamic nuclei, and the inferior olives5, 6. Additionally, moderate atrohpy of the cortex and basal ganglia were previously observed in FFI cases17. In this study, routine imaging of the brain did not reveal characteristic FFI features except for variable degrees of cerebral and cerebellar atrophy. Flourodeoxyglucose (18F) PET scan uncovered a profound bilateral thalamus with less pronounced cingular hypometabolism compared with the FFI signature image18, 19. In accordance with previously published data, the SPECT scan of Patient 1 and PET scan of Patient 2 in our study confirmed hypometabolism in the thalamus. Bar et al. previously reported that hypometabolism was confined to the thalamus in the earlier stages of the disease18.
Previous histopathological studies indicated severe neuronal loss in the thalamus17. Therefore, it was hypothesized that the dysregulation of the sleep-wake cycle is attributed to thalamic lesions20. However, animal studies showed that the thalamus is not critical or necessary for sleep-wake regulation21. Although thalamic strokes may cause hypersomnance, further analysis suggested that those strokes often extend to the hypothalamus and midbrain, which interferes with the ascending arousal projections. We speculate that basal ganglia-cortex circuits may also be involved in the regulation of the sleep-wake cycle. In this study, SPECT and PET scans revealed that basal ganglia and the cerebral cortex were affected in FFI. Furthermore, results obtained from rat models with induced lesions revealed that the basal ganglia are necessary for sleep architecture22, 23.
In conclusion, in this study, we analyzed the clinical features and sleep patterns of five FFI cases. We observed decreased sleep efficiency and disruption of the normal cyclic sleep organization. Nevertheless, this study had a few limitations like the small number of examined cases and the absence of histopathological data. In future studies, we will include a larger number of FFI cases, perform a detailed histopathological study, and use MRS, SPECT, and PET scans to elucidate the mechanism of FFI.
Materials and Methods
Ethics Statement
This study protocols outlined in this manuscript were approved by the Ethics Committee and local Institutional Review Board (IRB) of Xuan Wu Hospital, Capital Medical University, Beijing. All methods and experiments were performed in accordance with the relevant guidelines and regulations. All patients and healthy participants enrolled in the study signed an informed written consent specifically approved for this study prior to the study commence.
Subjects
This was a retrospective study that included five Chinese patients of Han descent who had been diagnosed with FFI and admitted to Xuan Wu Hospital (Beijing) from 2009 to 2014. The patients were diagnosed according to the FFI diagnosis criteria mentioned previously9. The clinical and phenotypic features of FFI were recorded for each patient at the time of admission.
Genetic and biochemical analysis for 14-3-3 protein epxression and PRNP gene mutation
Blood samples were obtained from all patients as well as several healthy control family members of the sibling patients #1 and #2 and patient’s #3 mother. PRNP gene mutation was detected in DNA isolated from the peripheral blood leukocytes as previously detailed16, 24. The 14-3-3 protein analysis was performed on cerebrospinal fluid samples by enzyme-linked immunosorbent assay (ELISA; Beijing TIANGEN) following the manufacturer’s protocol as described previously25.
EEG and brain imaging
EEG and brain magnetic resonance imaging (MRI) were performed for all five patients. In addition, Patient 1 underwent brain single photon emission computed tomography (SPECT) to detect blood flow perfusion and magnetic resonance spectrum (MRS). Patient 2 underwent a FDG positron emission tomography (PET) scan to illustrate the glucose metabolism of brain.
PSG analysis
Each patient underwent standard PSG (E-Series, Compumedics Limited, Abbotsford, Australia) study. The sleep-related laryngeal stridor, sleep breath disturbance, and involuntary movements were recorded. Sleep scoring was carried out for 30-second epochs according to AASM scoring criteria 2012. Next, sleep structure, sleep efficiency (total sleep time/total time in bed ×100%), arousal, periodic limb movement in sleep (PLMS) indices (AI and PLMS-I) (number of arousals and PLMS per hour of sleep), and the calculated AHI (apnea + hypopnea index) were evaluated.
Hypnogram analysis
Sleep stages (wake, N1, N2, N3, and REM sleep) were scored offline according to the American Academy of Sleep Medicine (AASM) scoring criteria by visual and spectral inspection of 30-second EEG epochs26.
Statistical analysis
In this study, the SPSS software was used to evaluate statistical significance. Differences in measured variables were assessed by using Student’s t test or Wilcoxon rank-sum test with nonparametric data. Results were considered statistically significant at P < 0.05.
Acknowledgements
This work was supported by grants from National Nature Science Foundation of China (NSFC) [grant no. 81571294 to Shuqin Zhan and 81470074 to Liyong Wu], and The Beijing Municipal Administration of Hospitals’ Youth Program [grant No. QML20160802 to Hui Lu].
Author Contributions
L.W., study concept and design, acquisition of data; H.L., analysis and interpretation of data, manuscript preparation and revision; X.W., acquisition of data, manuscript preparation and revision; J.L, manuscript preparation and revision; C.H., analysis and interpretation of PSG findings; J.Y., acquisition of data, manuscript revision; C.L., acquisition of data, manuscript revision; J.L., critical revision of manuscript for intellectual content; S.Z., study concept and design, analysis and interpretation of PSG findings; Y.W., critical revision of manuscript for intellectual content; J.J., study supervision and coordination. All authors have reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Liyong Wu and Hui Lu contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lugaresi E, et al. Fatal familial insomnia and dysautonomia with selective degeneration of thalamic nuclei. N Engl J Med. 1986;315(16):997–1003. doi: 10.1056/NEJM198610163151605. [DOI] [PubMed] [Google Scholar]
- 2.Montagna P. Fatal familial insomnia: a model disease in sleep physiopathology. Sleep Med Rev. 2005;9:339–353. doi: 10.1016/j.smrv.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Montagna P, Gambetti P, Cortelli P, Lugaresi E. Familial and sporadic fatal insomnia. Lancet Neurol. 2003;2:167–176. doi: 10.1016/S1474-4422(03)00323-5. [DOI] [PubMed] [Google Scholar]
- 4.Medori R, et al. Fatal familial insomnia, a prion disease with a mutation at codon 178 of the prion protein gene. N Engl J Med. 1992;326:444–449. doi: 10.1056/NEJM199202133260704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sforza E, et al. Sleep-wake cycle abnormalities in fatal familial insomnia. Evidence of the role of the thalamus in sleep regulation. Electroencephalogr Clin Neurophysiol. 1995;94:398–405. doi: 10.1016/0013-4694(94)00318-f. [DOI] [PubMed] [Google Scholar]
- 6.Montagna P. Fatal familial insomnia and the role of the thalamus in sleep regulation. Handb Clin Neurol. 2011;99:981–996. doi: 10.1016/B978-0-444-52007-4.00018-7. [DOI] [PubMed] [Google Scholar]
- 7.Montagna P, et al. Clinical features of fatal familial insomnia: phenotypic variability in relation to a polymorphism at codon 129 of the prion protein gene. Brain Pathol. 1998;8:515–520. doi: 10.1111/j.1750-3639.1998.tb00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tinuper P, et al. The thalamus participates in the regulation of the sleep-waking cycle. A clinico-pathological study in fatal familial thalamic degeneration. Electroencephalogr Clin Neurophysiol. 1989;73:117–123. doi: 10.1016/0013-4694(89)90190-9. [DOI] [PubMed] [Google Scholar]
- 9.Krasnianski A, et al. A proposal of new diagnostic pathway for fatal familial insomnia. J Neurol Neurosurg Psychiatry. 2014;85:654–659. doi: 10.1136/jnnp-2013-305978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarranz JJ, et al. Phenotypic variability in familial prion diseases due to the D178N mutation. J Neurol Neurosurg Psychiatry. 2005;76:1491–1496. doi: 10.1136/jnnp.2004.056606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu S, et al. Early onset fatal familial insomnia with rapid progression in a Chinese family line. J Neurol. 2007;254:1300–1301. doi: 10.1007/s00415-006-0517-0. [DOI] [PubMed] [Google Scholar]
- 12.Macchi G, et al. Diffuse thalamic degeneration in fatal familial insomnia. A morphometric study. Brain Res. 1997;771:154–158. doi: 10.1016/S0006-8993(97)00902-5. [DOI] [PubMed] [Google Scholar]
- 13.Isono S, et al. Pathogenesis of laryngeal narrowing in patients with multiple system atrophy. J Physiol. 2001;536:237–249. doi: 10.1111/j.1469-7793.2001.t01-1-00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsden CD, Obeso JA, Zarranz JJ, Lang AE. The anatomical basis of symptomatic hemidystonia. Brain. 1985;108(Pt 2):463–483. doi: 10.1093/brain/108.2.463. [DOI] [PubMed] [Google Scholar]
- 15.Lee MS, Marsden CD. Movement disorders following lesions of the thalamus or subthalamic region. Mov Disord. 1994;9:493–507. doi: 10.1002/mds.870090502. [DOI] [PubMed] [Google Scholar]
- 16.Schulz-Schaeffer WJ, et al. The paraffin-embedded tissue blot detects PrP(Sc) early in the incubation time in prion diseases. Am J Pathol. 2000;156:51–56. doi: 10.1016/S0002-9440(10)64705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manetto V, et al. Fatal familial insomnia: clinical and pathologic study of five new cases. Neurology. 1992;42:312–319. doi: 10.1212/WNL.42.2.312. [DOI] [PubMed] [Google Scholar]
- 18.Bär KJ, et al. Serial positron emission tomographic findings in an atypical presentation of fatal familial insomnia. Arch Neurol. 2002;59:1815–1818. doi: 10.1001/archneur.59.11.1815. [DOI] [PubMed] [Google Scholar]
- 19.Cortelli P, et al. Pre-symptomatic diagnosis in fatal familial insomnia: serial neurophysiological and 18FDG-PET studies. Brain. 2006;129:668–675. doi: 10.1093/brain/awl003. [DOI] [PubMed] [Google Scholar]
- 20.Lugaresi E. The thalamus and insomnia. Neurology. 1992;42:28–33. [PubMed] [Google Scholar]
- 21.Fuller PM, et al. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519:933–956. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu MH, Vetrivelan R, Fuller PM, Lu J. Basal ganglia control of sleep-wake behavior and cortical activation. Eur J Neurosci. 2010;31:499–507. doi: 10.1111/j.1460-9568.2009.07062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu MH, Yao QL, Vetrivelan R, Chen MC, Lu J. Nigrostriatal Dopamine Acting on Globus Pallidus Regulates Sleep. Cereb Cortex. 2016;26:1430–1439. doi: 10.1093/cercor/bhu241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kretzschmar HA, Ironside JW, DeArmond SJ, Tateishi J. Diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Arch Neurol. 1996;53:913–920. doi: 10.1001/archneur.1996.00550090125018. [DOI] [PubMed] [Google Scholar]
- 25.Zerr I, et al. Detection of 14-3-3 protein in the cerebrospinal fluid supports the diagnosis of Creutzfeldt-Jakob disease. Ann Neurol. 1998;43:32–40. doi: 10.1002/ana.410430109. [DOI] [PubMed] [Google Scholar]
- 26.Ruehland WR, et al. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32:150–157. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]


