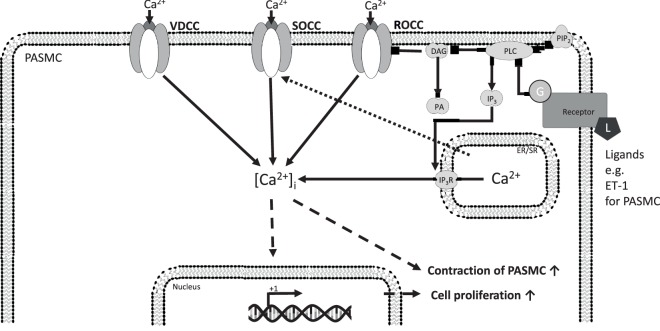
Figure 1.
[Ca2+]i homeostasis regulation in precapillary pulmonary arterial smooth muscle cells (PASMCs) and ECs. Ca2+ enters cells from extracellular fluid through L-type voltage-dependent calcium channels or non-selective cation channels, which can be divided into SOCCs and ROCCs. The initiation of ROCC-mediated Ca2+-influx from the extracellular space is thought to be induced by ligand-activated G-protein coupled receptors, starting a PLC-mediated hydrolyzation of PIP2 to IP3 and DAG. DAG regulates the activity of ROCC to induce receptor-operated Ca2+ entry, whereas IP3 generation induces depletion of the intracellular Ca2+ stores in the endoplasmic reticulum, leading to induction of store-operated Ca2+ entry. The increased [Ca2+]i drives different cellular responses. Ca2+, calcium ion; [Ca2+]i, intracellular Ca2+ concentration; ROCC, receptor-operated calcium channel; SOCC, store-operated calcium channel; VDCC, L-type voltage-dependent calcium channel; DAG, diacylglycerol; DAGK, DAG kinase; EC, endothelial cell; ER/SR, endoplasmic/sarcoplasmic reticulum; IP3, inositol trisphosphate; IP3R, inositol trisphosphate receptor; L, ligand; PA, phosphatidic acid; PASMC, precapillary pulmonary arterial smooth muscle cells; PIP2, phosphatidylinositol 4,5-bisphosphate; PLC, phospholipase C; VEGF, vascular endothelial growth factor; solid arrows indicate direct interactions; dotted arrows illustrate indirect interactions.